Human vascular endothelial cell culture solution and culture method
A vascular endothelial and cell culture technology, which is applied in the direction of vascular endothelial cells, cell dissociation methods, cell culture active agents, etc., can solve the problems of secret formula and high price, and achieve the effects of less pollution, convenient preparation, and promotion of cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
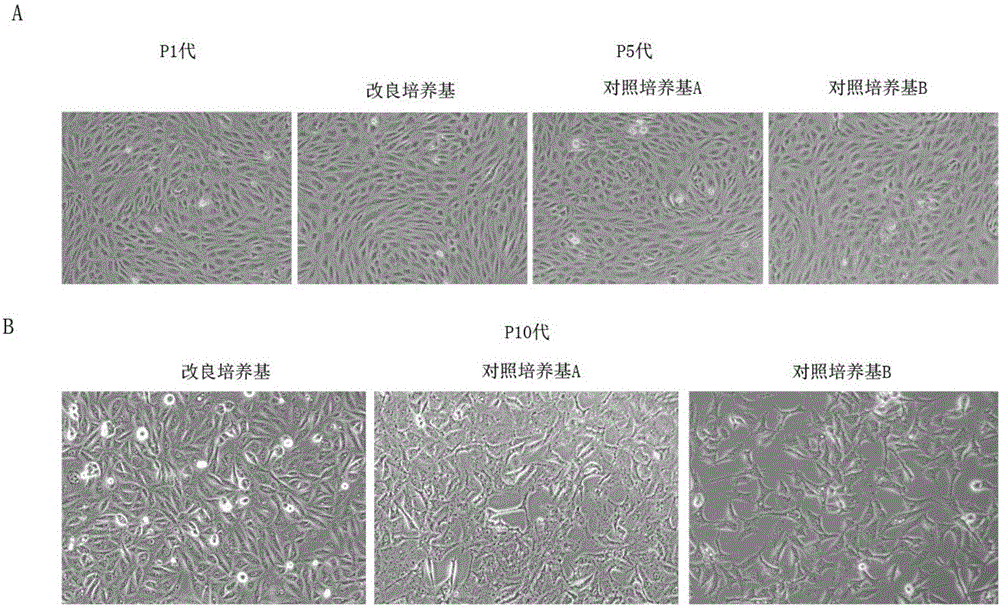
[0063] The present embodiment adopts the culture solution of the present invention, the M199 complete medium containing 10% fetal bovine serum and the ECM serum-containing medium of sciencecell to carry out comparative experiments. The three culture solutions are respectively:
[0064] Culture solution (improved culture medium) of the present invention:
[0065] On the basis of every 100ml DMEM / F12 medium, add the following components: 10ml fetal bovine serum, 100U / ml penicillin, 100mg / ml streptomycin, 1.25ml L-glutamine, 90μg / ml heparin, 5ng / ml ml human recombinant epidermal growth factor, 10ng / ml human recombinant fibroblast growth factor, 0.5ng / ml vascular endothelial growth factor, 20ng / ml insulin-like growth factor, 1μg / ml vitamin C, 1μg / ml cortisol. 100 ng / ml cholera toxin, 10 μM aminoethanol, 100 pM phosphoethanolamine.
[0066] Control medium A: M199 complete medium containing 10% fetal bovine serum
[0067] Control Medium B: Sciencecell’s ECM Serum-Containing Medium ...
Embodiment 2
[0076] Recovery, subculture, cryopreservation and rethrowing of human microvascular endothelial cells HMEC-1. See Example 1 for details of the three cell culture media used.
[0077] First, put the improved culture medium of the present invention, control culture medium A and control culture medium B three kinds of culture medium into 3 petri dishes according to the medium consumption of 1ml / 5cm, put into 37 ± 1 ℃, 5% CO 2 , Preheat in an incubator with a humidity of 90±2% for 30 minutes.
[0078] Then three cryopreservation tubes of the same batch of frozen HMEC-1 cells were taken out from the liquid nitrogen, and quickly put into a 37°C water bath for thawing. After the cryopreservation solution is completely thawed, transfer 1ml of the frozen cells in the cryopreservation tube to a centrifuge tube containing 9ml of serum-free medium, centrifuge at 800rpm / min for 5min, and resuspend the cell pellet with 1ml of the corresponding complete medium Then add the cell suspension ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com