A kind of substituted tetrahydrofuran water-soluble derivative and its application
A technology of drugs and compounds, applied in the field of medicinal chemistry, can solve problems such as poor water solubility, inconvenience and pain of patients, and achieve the effects of less vascular irritation, high bioavailability, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of MJ10821
[0045] (1) Synthesis of intermediate M21
[0046]
[0047]Dissolve Cbz-Val-Ala-OH (322mg, 1mmol) in 3ml N,N-dimethylformamide (DMF), cool down to -5°C, add DIC (63.1mg, 0.5mmol) under stirring, and react at room temperature 30min, continue to cool down to -5°C, add II3 (350mg, 0.5mmol) in 2ml DMF solution, triethylamine (60.7mg, 0.6mmol), catalytic amount of 4-dimethylaminopyridine (DMAP), and react at room temperature for 4h, After the reaction was completed, it was poured into 15ml of water, extracted 3 times with 15ml of ethyl acetate, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was spin-dried to obtain a yellow solid, which was separated by silica gel column chromatography to obtain 281 mg of off-white powder (M21). Yield 56%. MS(m / z): 1005.5[M+1] + .
[0048] (2) Synthesis of MJ10821
[0049]
[0050] Dissolve M21 (251mg, 0.25mmol) in 3ml of methanol, add 200mg of 5% Pd / C...
Embodiment 2
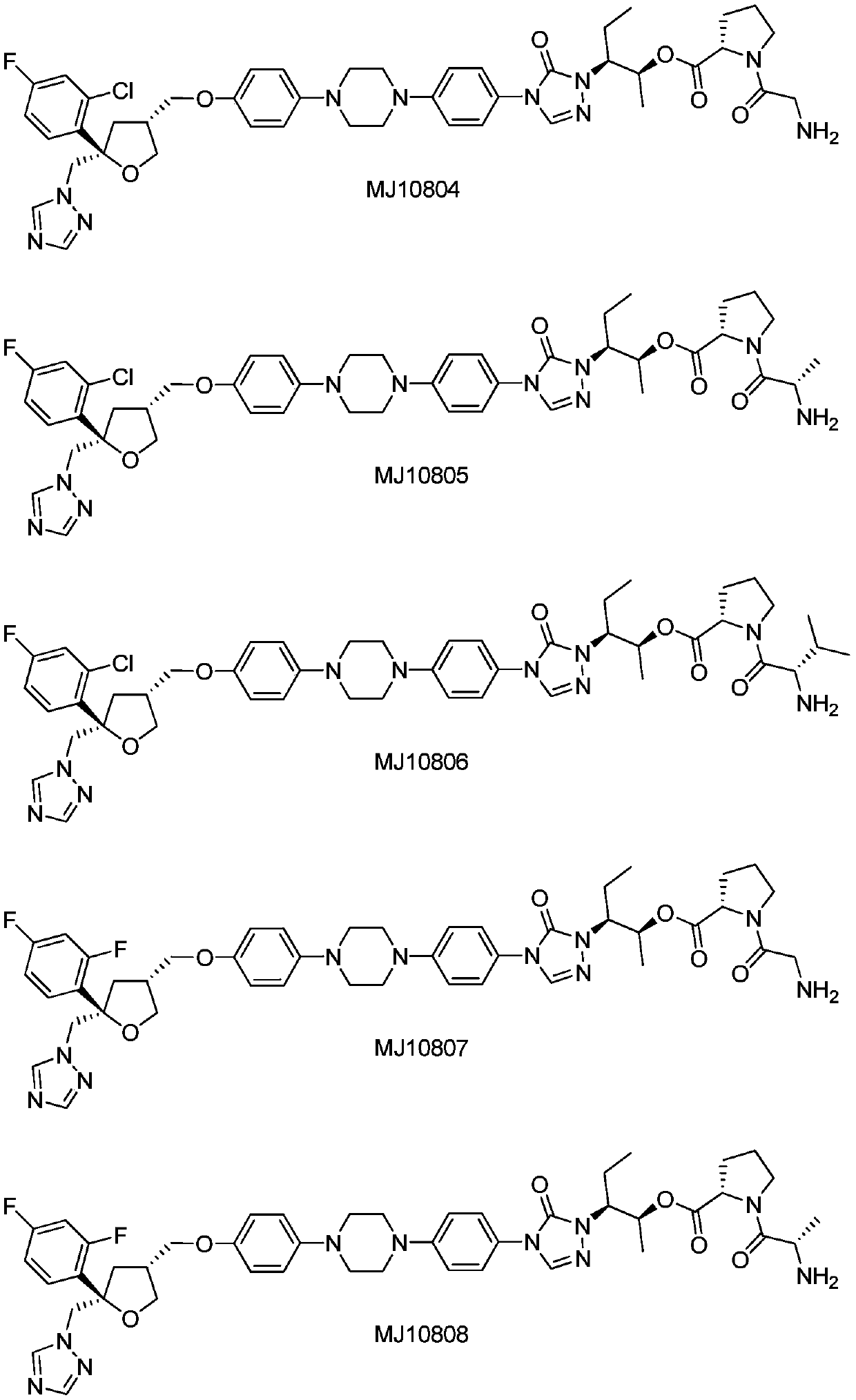
[0051] Embodiment 2: the synthesis of MJ10807
[0052] Refer to the synthesis of MJ10821, the difference is that Cbz-Gly-Pro-OH and II3 are selected as starting materials, and after condensation and deprotection, MJ10807 is finally obtained. MS(m / z): 855.4[M+1] + .
Embodiment 3
[0053] Embodiment 3: the synthesis of MJ10808
[0054] Refer to the synthesis of MJ10821, the difference is that Cbz-Ala-Pro-OH and II3 are selected as starting materials, and after condensation and deprotection, MJ10808 is finally obtained. MS(m / z): 869.4[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com