Chimeric antigen receptor and gene and recombinant expression vector thereof, carher1-nkt cell and preparation method and application thereof
A chimeric antigen receptor, NKT cell technology, applied in the field of tumor biological products, achieves good industrial application prospects, enhances ability, and enhances the effect of specific killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137-CD3ζ is not particularly limited, and can be various methods that can be imagined by those skilled in the art. Preferably, the lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137 -The preparation method of CD3ζ comprises the following steps:
[0031] (1) The hinge region and transmembrane region of CD8, the intracellular signaling domain of CD137 and the intracellular signaling domain of CD3ζ were respectively amplified from NKT cell cDNA, and cloned into the vector pWPXL-GFP to construct pWPXL-CD8 - CD137-CD3ζ;
[0032] (2) Synthesize the nucleotide sequence encoding rat growth hormone signal peptide and HER1ScFv, and clone it into pWPXL-CD8-CD137-CD3ζ, and obtain the correct sequence of pWPXL-HER1ScFv-CD8-CD137-CD3ζ after sequencing verification.
[0033] In step (1), there is no particular limitation on the methods for respectively amplifying the hinge region and transmembrane regi...
Embodiment 1
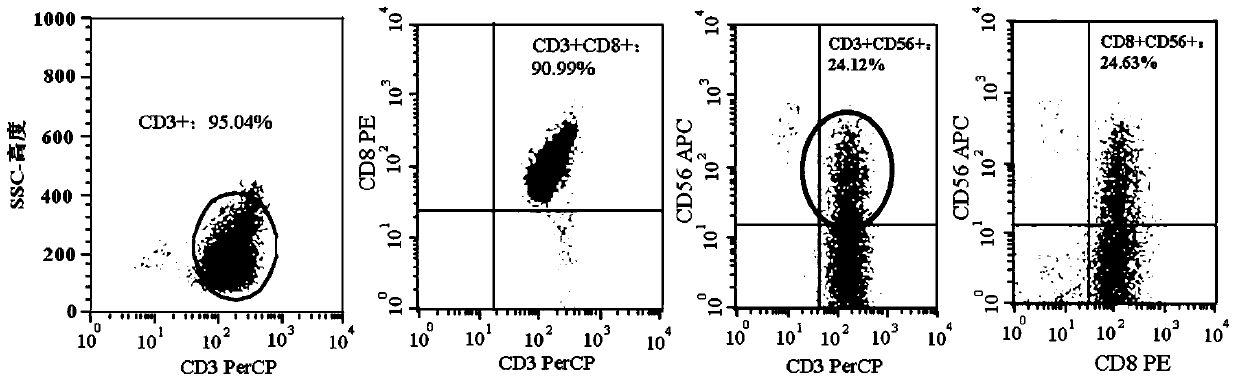
[0084] The preparation of embodiment 1 NKT cell
[0085] (1) Take human venous blood in a vacuum tube containing heparin. Mononuclear cells (PBMCs) were obtained by density gradient centrifugation using lymphocyte separation medium.
[0086] (2) After PBMCs were washed three times, the final concentration of cells was adjusted to 2×10 by using NKT cell culture medium GT-T551 containing 0.6 volume % human autologous serum. 6 cells / mL; the cells were inoculated on a 75 cm 2 in cell culture flasks. Then add recombinant human interleukin-2 with a final concentration of 500U / mL, 50ng / ml CD3 monoclonal antibody and 50ng / mL recombinant human interleukin-15 to the culture medium, at 37°C and saturated humidity of 5% CO 2 cultured in an incubator.
[0087] (3) On the 4th day of culture, transfer the cells to an uncoated culture flask, add NKT cell culture medium GT-T551 every 2 days according to the number of cell growth, and control the cell concentration to 1×10 8 cells / mL, and ...
Embodiment 2
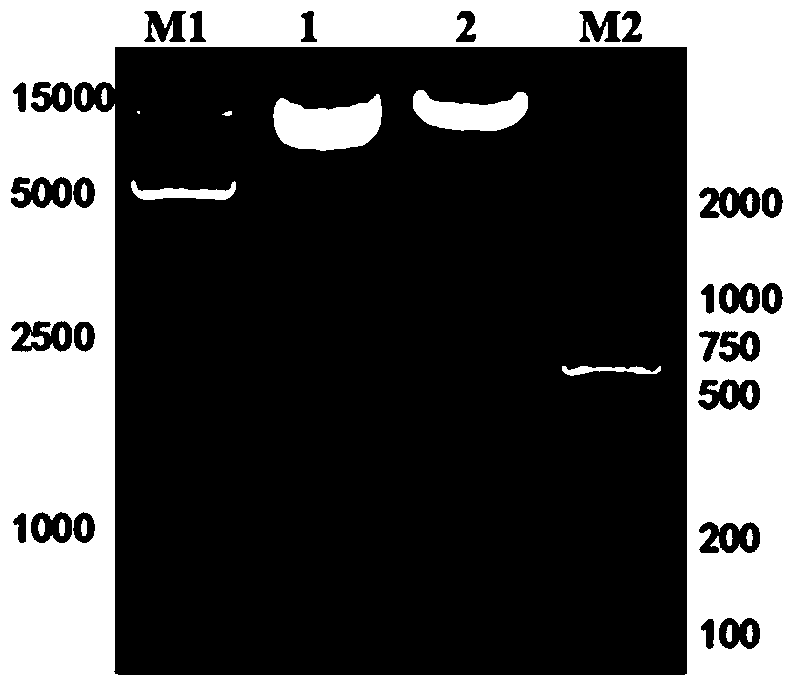
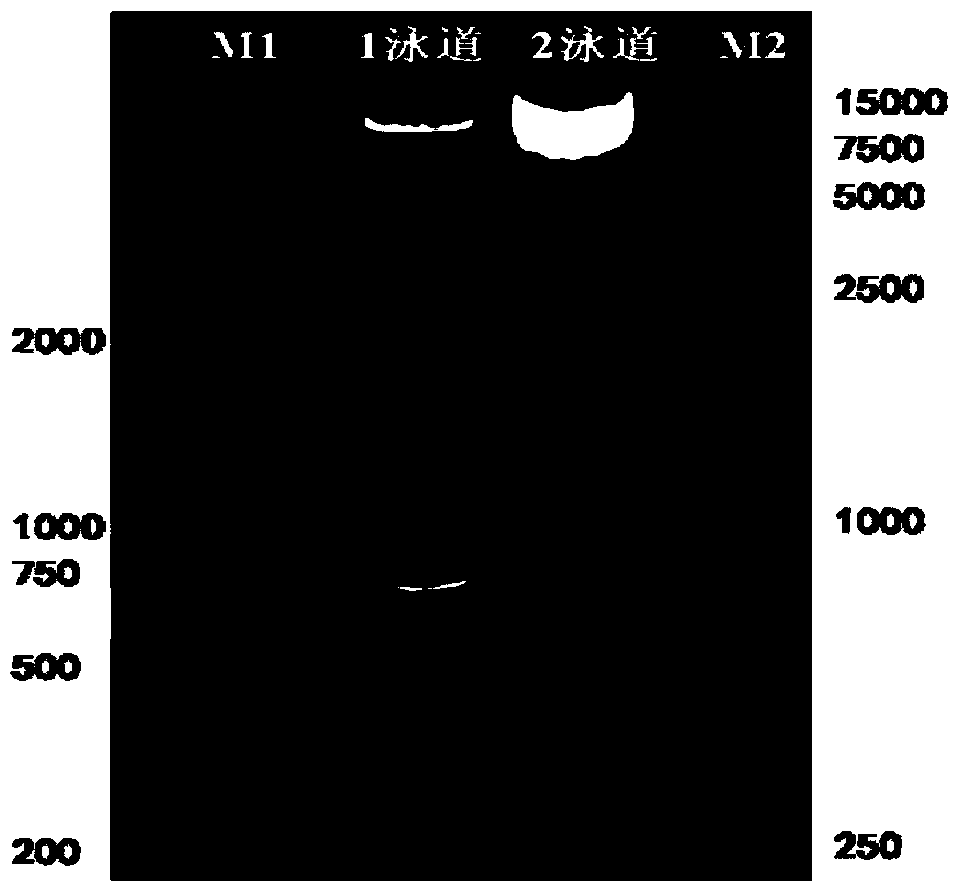
[0088] Example 2 Construction of lentiviral expression vector pWPXL-HER1ScFv-CD8-CD137-CD3ζ
[0089] (1) Preparation of NKT cell cDNA
[0090] The NKT cells cultured in Example 1 were centrifuged to precipitate, and the total RNA of the cells was extracted with RNAiso Reagent, a total RNA extraction kit, and stored at -80°C for future use. Extracted total RNA with RevertAid Reverse Transcription Kit TM NKT cell cDNA was obtained by reverse transcription with the First StrandcDNA Synthesis Kit, and stored at -20°C for future use.
[0091] (2) Preparation of lentiviral plasmid pWPXL-CD8-CD137-CD3ζ
[0092] The following primer sequences were designed and synthesized (wherein, the underline marks are protected bases, and the square boxes are enzyme cleavage sites):
[0093] P1 (SEQ ID NO.11): GATC CTGAGCAACTCCATCATGTACTTC
[0094] MluI
[0095] P2 (SEQ ID NO.12): GATC GCAGTAAAGGGTGATAACCAGTGA
[0096] BglII
[0097] P3 (SEQ ID NO.13): GATC AAACGGGGCAGAAAGAAACT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com