Method for converting and purifying 4-androstenedione from mother solutions of allyl ester substances and ketal substances
A technology of androstenedione and ketal, which is applied in the field of purification and preparation of 4-androstenedione, can solve the problems of low product purity and yield, high raw material consumption, long reaction route, etc., and achieve product purity and High yield, low dosage, and the effect of preventing intermediate side reaction products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
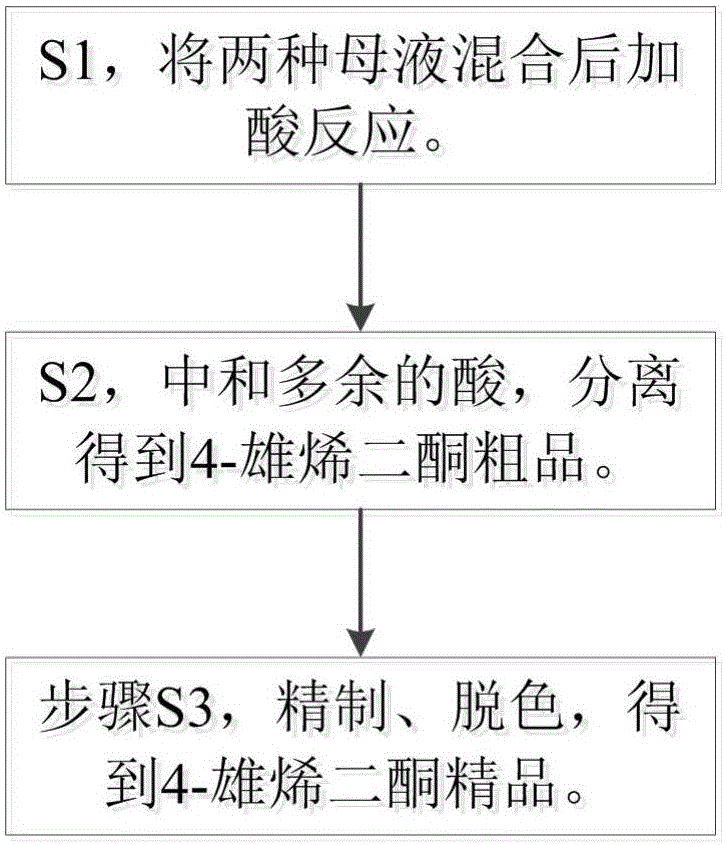
[0025] S1, 10g of the enester mother liquor obtained in the preparation process of dehydroepiandrosterone, and 10g of the ketal mother liquor obtained in the preparation process of dehydroepiandrosterone are heated and dissolved in 150g methanol, and the concentration of 10g is added to 10mol / L hydrochloric acid, react at 45°C for 2.5h, take a sample for TLC detection until the reaction is complete;
[0026] S2, adjusting the pH of the solution to 6 with an aqueous solution of sodium carbonate, adding 10 times the volume of water to precipitate crystals, centrifuging, washing with water, pulverizing, and drying to obtain crude 4-androstenedione;
[0027] Step S3: Mix 50 g of the crude 4-androstenedione obtained in step S2 with 500 g of methanol, add 2.5 g of activated carbon, reflux at 70-90° C. for 1 hour, and concentrate the crystals by suction filtration to obtain fine 4-androstenedione.
[0028] Under the detection of HPLC, the mass percent content of 4-androstenedione in ...
Embodiment 2
[0031] S1, 10g of the enester mother liquor obtained in the preparation process of dehydroepiandrosterone, and 30g of the ketal mother liquor obtained in the preparation process of dehydroepiandrosterone are heated and dissolved in 150g methanol, and 20g of concentration is added 10mol / L hydrochloric acid, react at 50°C for 3.5h, take a sample for TLC detection until the reaction is complete;
[0032] S2, adjusting the pH of the solution to 7 with an aqueous solution of sodium bicarbonate, adding 10 times the volume of water to precipitate crystals, centrifuging, washing with water, pulverizing, and drying to obtain crude 4-androstenedione;
[0033] Step S3, mixing 50 g of the crude product of 4-androstenedione obtained in step S2 with 500 g of ethyl acetate, adding 5 g of activated carbon, refluxing at 70-90° C. for 0.5 h, suctioning and concentrating the crystals to obtain the refined product of 4-androstenedione .
[0034] Under the detection of HPLC, the mass percent cont...
Embodiment 3
[0037] S1, 10g of enester mother liquor obtained in the preparation process of dehydroepiandrosterone, and 50g of ketal mother liquor obtained in the preparation process of dehydroepiandrosterone are heated and dissolved in 200g methanol, and 20g concentration is added 10mol / L hydrochloric acid, react at 55°C for 4.5h, take a sample for TLC detection until the reaction is complete;
[0038] S2, adjust the pH of the solution to 7 with aqueous sodium hydroxide solution, add 10 times the volume of water to precipitate crystals, centrifuge, wash with water, pulverize, and dry to obtain crude 4-androstenedione;
[0039] Step S3: Mix 50 g of the crude 4-androstenedione obtained in step S2 with 500 g of ethanol, add 25 g of activated carbon, reflux at 70-90° C. for 1.5 h, and concentrate the crystals by suction filtration to obtain fine 4-androstenedione.
[0040] Under the detection of HPLC, the mass percent content of 4-androstenedione in the obtained 4-androstenedione refined prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com