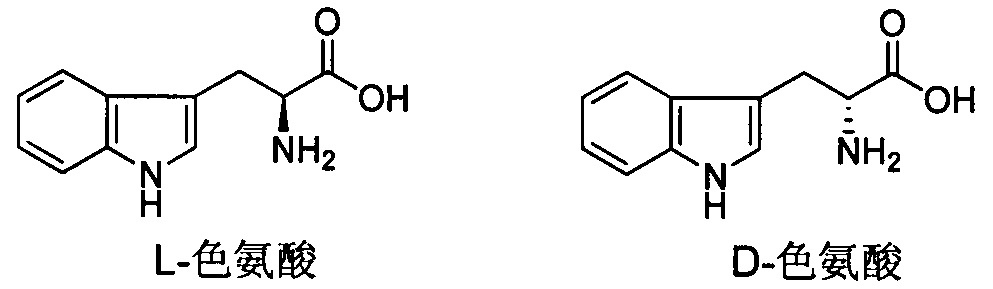
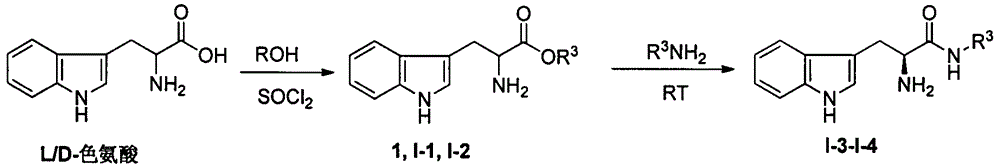
Tryptophan derivative, preparing method and application in preventing and treating plant viruses, killing bacteria and killing insects
A technology of tryptophan and its derivatives, applied in the field of pesticides, can solve the problems of poor therapeutic effect and achieve excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Synthesis of (S)-2-amino-3-(1H-indole-3)-propionic acid methyl ester (1)
[0072]
[0073] Add 10 g (49.02 mmol) of L-tryptophan and 150 mL of methanol into a 250 mL single-necked round bottom flask, add 10 mL of thionyl chloride dropwise with stirring, and then stir under heating and reflux for 4 hours. TLC monitors that the reaction is complete and the solution is removed. The obtained solid is washed with saturated aqueous sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, filtered, and removed to obtain 9.16 g of a brown solid with a melting point of 90-91°C and a yield of 86%. 1 H NMR(300MHz, CDCl 3 )δ8.47(s, 1H, Ar-NH), 7.60(d, 3 J HH =7.8Hz, 1H, Ar-H), 7.32(d, 3 J HH = 8.1 Hz, 1H, Ar-H), 7.18 (t, 3 J HH =7.2Hz, 1H, Ar-H), 7.11(t, 3 J HH =7.2Hz, 1H, Ar-H), 7.00(s, 1H, Ar-H), 3.82(dd, 3 J HH =4.8Hz, 2 J HH 7.5Hz, 1H, CH), 3.71(s, 3H, CH) 3 ), 3.28(dd, 3 J HH =4.5Hz, 2 J HH =14.4Hz, 1H, CH 2 ), 3.04(dd, 3 J HH =7.5H...
Embodiment 2
[0077] Example 2: Synthesis of (S)-2-amino-N-butyl-3-(1H-indole-3-)propionamide (I-3)
[0078]
[0079] Add 0.40g (1.83mmol) methyl ester 1,10mL n-butylamine to a 25mL single-necked round-bottom flask, stir at room temperature for 2d, desolvate, add dichloromethane / water liquid extraction, dry the organic phase with anhydrous sodium sulfate, filter, Dissolve and drain to obtain 0.33 g of yellow viscous liquid with a yield of 70%. 1 H NMR(300MHz, CDCl 3 )δ8.14(s, 1H, Ar-NH), 7.69(d, 3 J HH =7.8Hz, 1H, Ar-H), 7.38(d, 3 J HH =7.8Hz, 1H, Ar-H), 7.21(t, 3 J HH =7.5Hz, 1H, Ar-H), 7.13(t, 3 J HH =7.5Hz, 1H, Ar-H), 7.08(s, 1H, Ar-H), 3.71(dd, 3 J HH =9.0Hz, 3.9Hz, 1H, CH), 3.40(dd, 3 J HH =3.9Hz, 2 J HH =14.4Hz, 1H, CH 2 ), 3.25(q, 3 J HH =6.6Hz, 1H, CH 2 ), 2.91(dd, 3 J HH =9.0Hz, 2 J HH =14.4Hz, 1H, CH 2 ), 1.57-1.20(m, 4H, CH 2 CH 2 ), 0.91(t, 3 J HH =7.5Hz, 3H, CH 3 ); 13 C NMR(100MHz, CDCl 3 )δ174.7, 136.4, 127.5, 123.1, 122.2, 119.5, 119.0, 111.8, 111.3, 55.7, 38.8, 31.6, ...
Embodiment 3
[0080] Example 3: (S)-1-(2-hydroxyethylamino)-3-(1H-indole-3-)-1-oxopropan-2-ammonium hydrochloride (I-4) synthesis
[0081]
[0082] Add 0.50g (2.29mmol) of methyl ester 1,10mL ethanolamine to a 25mL single-necked round-bottom flask, stir at room temperature for 2.5d, transfer the reaction solution to a 100mL single-necked flask, add enough ethanol to desolvate, and dissolve the obtained solid with dichloromethane HCl gas was passed into the liquid, a yellow solid was precipitated, cooled, filtered, and the mother liquor was desolvated. DCM / MeOH=5:1 (v / v) was used as the eluent for column chromatography to obtain a yellow solid 0.32 g. The yield is 49%, and the melting point is 118-120°C. 1 H NMR(400MHz, DMSO-d 6 )δ10.92(s, 1H, NH), 8.03(t, 3 J HH =4.8Hz, 1H, O=C-NH), 7.57(d, 3 J HH =8.0Hz, 1H, Ar-H), 7.34(d, 3 J HH =8.0Hz, 1H, Ar-H), 7.17(d, 4 J HH =1.6Hz, 1H, Ar-H), 7.06(t, 3 J HH =7.6Hz, 1H, Ar-H), 6.97(t, 3 J HH =7.6Hz, 1H, Ar-H), 4.75 (br, 1H, OH), 3.49 (dd, 3 J HH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com