Primers, kit and method for qualitatively detecting leukaemia fusion genes
A technology for detecting primers and fusion genes, which is applied in the field of genetic engineering and can solve the problems of increasing the probability of multiple pairs of primers interacting and forming primer-dimers, failing to amplify specific target products, and reducing the specificity and efficiency of PCR reactions. , to achieve the effect of saving reagent consumption, reducing detection cost, and improving specificity and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Utilize fusion gene positive cell line and negative cell line to detect the specificity of detection system of the present invention
[0040] In this example, the feasibility and specificity of the detection method of the present invention are verified by determining the K562 positive cell line containing the BCR-ABL1 fusion gene, the Kasumi cell line containing the AML1-ETO fusion gene, and the HL60 negative cell line without the fusion gene. Among them, K562 cell line, Kasumi cell line, and HL60 cell line were all purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd.
[0041] The specific detection method of the present embodiment is as follows:
[0042] 1. Cell culture
[0043] Cultivate K562 cell line, Kasumi cell line and HL60 cell line according to the standard operation of cell culture at 37°C, 5% CO 2 Cultured in an incubator.
[0044] 2. Nucleic acid extraction
[0045] It is recommended to use TIANGEN RNAprep Pure Blood Kit, follo...
Embodiment 2
[0059] Embodiment 2: Utilize fusion gene positive cell line and negative cell line to detect the sensitivity of detection system of the present invention
[0060] In this embodiment, the sensitivity of the detection system of the present invention is also verified by determining the K562 positive cell line containing the BCR-ABL1 fusion gene, the Kasumi cell line containing the AML1-ETO fusion gene, and the HL60 negative cell line without the fusion gene. The specific detection method is as follows:
[0061] K562 cell line (positive cell line containing BCR-ABL1 fusion gene) and Kasumi cell line (positive cell line containing AML-ETO fusion gene) were carried out with HL-60 cell line (negative cell line without fusion gene) respectively. Dilution of 100%, 10%, 1%, 1‰, 0.5‰, 0.25‰, 0.125‰ (the ratio of positive cell line to negative cell line after dilution), extract RNA with diluted mixed cell line and reverse transcribe into cDNA , for nested RT-PCR detection.
[0062] Usin...
Embodiment 3
[0064] Example 3: Detection of clinical samples using the detection system of the present invention
[0065] In this example, 150 peripheral blood or bone marrow blood samples were collected from clinical leukemia patients, and the qualitative detection of 43 fusion genes was carried out according to the detection system of the present invention. Among them, 25 cases of clinical samples were detected as fusion gene positive by multiple nested RT-PCR of the present invention, and 125 cases were fusion gene negative.
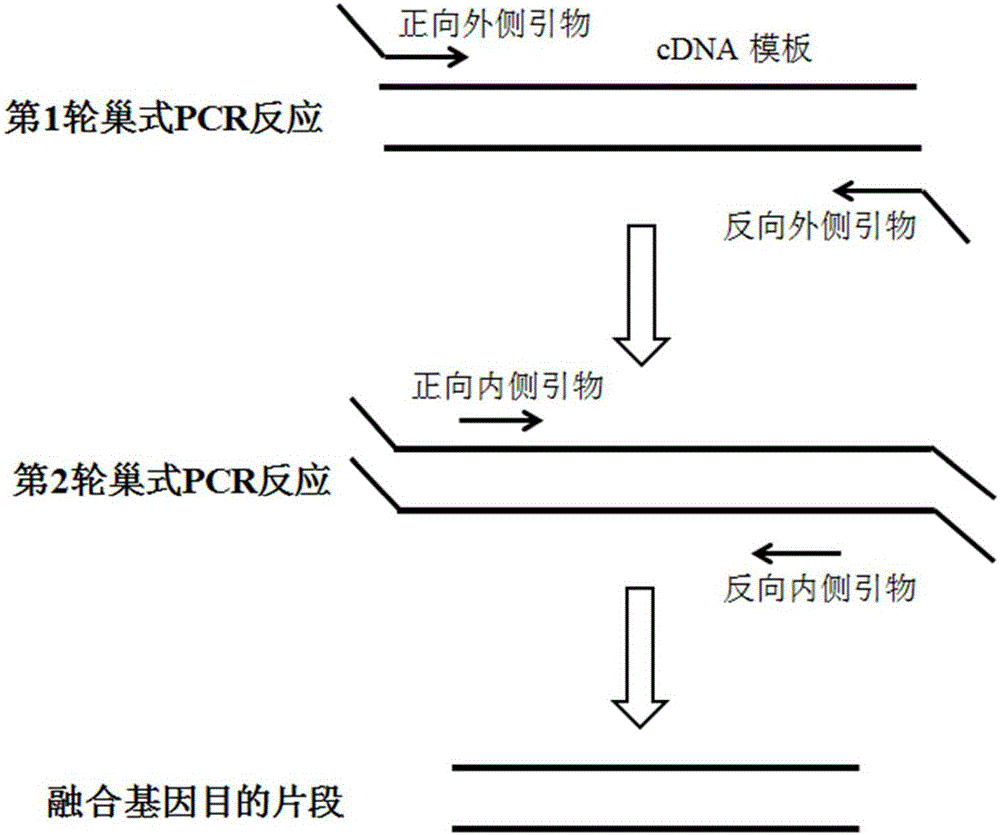
[0066] Using the multiplex nested RT-PCR detection system of the present invention to detect clinical samples, the operation steps include leukocyte RNA extraction from blood samples, RNA reverse transcription into cDNA, first round reaction of nested PCR, second round reaction of nested PCR, separation PCR Reaction products, agarose gel electrophoresis. The specific operation method is the same as that of Embodiment 1 of the present invention. For the results o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com