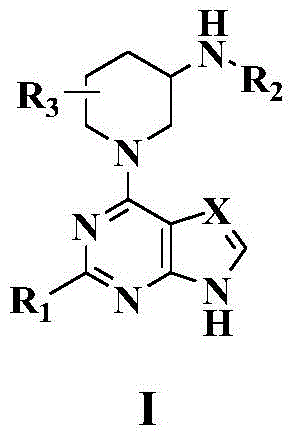
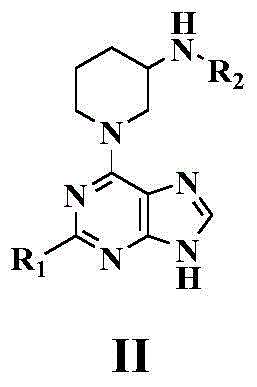
1-(pyrimidine-4-yl)-3-aminopiperdine derivative and its preparation method and use
A technology of aminopiperidine and its derivatives, applied in the field of 1-3-aminopiperidine derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1 Synthesis of (R)-tert-butyl (1-(9H-purin-6-yl)piperidin-3-yl) carbamate (Compound 1)
[0091]
[0092] At room temperature, 6-chloropurine (1 mmol) and R-3-Boc aminopiperidine (1.2 mmol) were added to methanol. After stirring for 5 minutes, the temperature was slowly raised to 50 degrees Celsius. After stirring for 7 hours, a precipitate precipitated and the reaction was stopped. After cooling to room temperature, the reaction solution was filtered, the filter cake was washed with a little methanol, the filter cake was collected and drained to obtain a crude product. The crude product was purified by column chromatography to obtain the final target compound 1, yield: 42%.
[0093] 1 H NMR(400MHz, DMSO-d6): δ12.98(s,1H), 8.20(s,1H), 8.11(s,1H), 6.94(d,1H,J=6.4Hz), 5.16-5.04(m , 2H), 3.34 (s, 1H), 3.23-3.05 (m, 2H), 1.90-1.79 (m, 2H), 1.50 (t, 2H, J=4.2 Hz), 1.39 (s, 9H). MS(ESI),m / z:317.4[M-H] - .
Embodiment 2
[0094] Example 2 Synthesis of (R)-N-(1-(9H-purin-6-yl)piperidin-3-yl)acetamide (Compound 2)
[0095]
[0096] Compound 1 (1 mmol) was added to dichloromethane (2 mL) and trifluoroacetic acid (2 mL), stirred at room temperature for 4 hours, and the reaction was monitored by thin layer chromatography. When the reaction is complete, the reaction solution is spin-dried, and ether is added to precipitate a precipitate, which is filtered to obtain the trifluoroacetate intermediate of compound 1. Add the trifluoroacetate (1mmol) of compound 1 to tetrahydrofuran, add triethylamine (3mmol), slowly add acetyl chloride (1.2mmol) under an ice bath, and react at room temperature. After the reaction is complete, spin dry the solution at low temperature. Add methanol to dissolve and purify by column chromatography to obtain compound 2, yield: 40%.
[0097] 1 H NMR (400MHz, DMSO-d6): δ10.01 (s, 1H), 8.19 (s, 1H), 8.10 (s, 1H), 7.91 (d, 1H, J = 8.0 Hz), 5.34-4.66 (m ,2H),3.72-3.71(m,1H),3.43-3.36...
Embodiment 3
[0098] Example 3 Synthesis of (R)-N-(1-(9H-purin-6-yl)piperidin-3-yl)palmitamide (Compound 3)
[0099]
[0100] Compound 1 (1 mmol) was added to dichloromethane (2 mL) and trifluoroacetic acid (2 mL), stirred at room temperature for 4 hours, and the reaction was monitored by thin layer chromatography. When the reaction is complete, the reaction solution is spin-dried, and ether is added to precipitate a precipitate, which is filtered to obtain the trifluoroacetate intermediate of compound 1. Add the trifluoroacetate (1mmol) of compound 1 to tetrahydrofuran, add triethylamine (3mmol), slowly add pivaloyl chloride (1.2mmol) under ice bath, and react at room temperature. After the reaction is complete, spin dry the solution at low temperature , Adding methanol to dissolve and purifying by column chromatography to obtain compound 3, yield: 35%.
[0101] 1 H NMR (400MHz, DMSO-d6): δ8.37 (s, 1H), 7.98 (s, 1H), 6.16 (d, 1H, J = 4.2Hz), 4.64-4.28 (m, 2H), 4.23-4.01 (m,3H),2.00-1.89(m,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com