Intracranial drug eluting stent system and preparation method thereof
A technology of eluting stents and drugs, which is applied in the field of special drug-eluting stent systems, can solve the problems of increased lumen loss in the late stage, reduce restenosis catch-up problems, improve long-term safety, and achieve the effect of vascular function restoration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of intracranial drug-eluting stent
[0052] 1) Preparation of stent base coating
[0053] Use dimethylformamide as a solvent to dissolve n-butyl methacrylate monomer, add sodium nitrate as an electrolyte to increase the conductivity of the solvent, and stir for 30 minutes.
[0054] Table 1. Solution preparation ratio
[0055]
[0056] After the metal stent (the stent material is 316L stainless steel (Germany Euroflex)) was cleaned and dried, it was placed in the reaction vessel containing the above solution, and the polymerization of the stent base coating was completed by applying current and voltage scanning to the solution.
[0057] Electrochemical conditions: voltage: 20V; reaction time: 120 minutes; nitrogen pressure: 2 atmospheres.
[0058] After the reaction, the stent was placed in a vacuum oven to dry.
[0059] 2) Drug coating preparation
[0060] Dissolve polylactic acid polyglycolic acid particles (PLGA, 50 / 50, Mn=90,000-120,000, ...
Embodiment 2
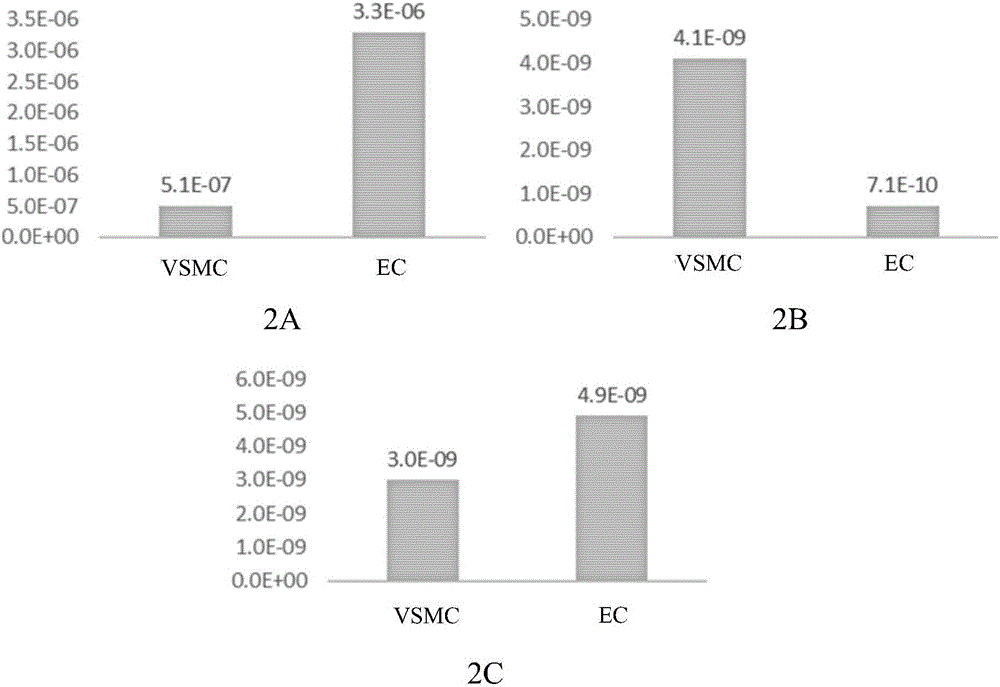
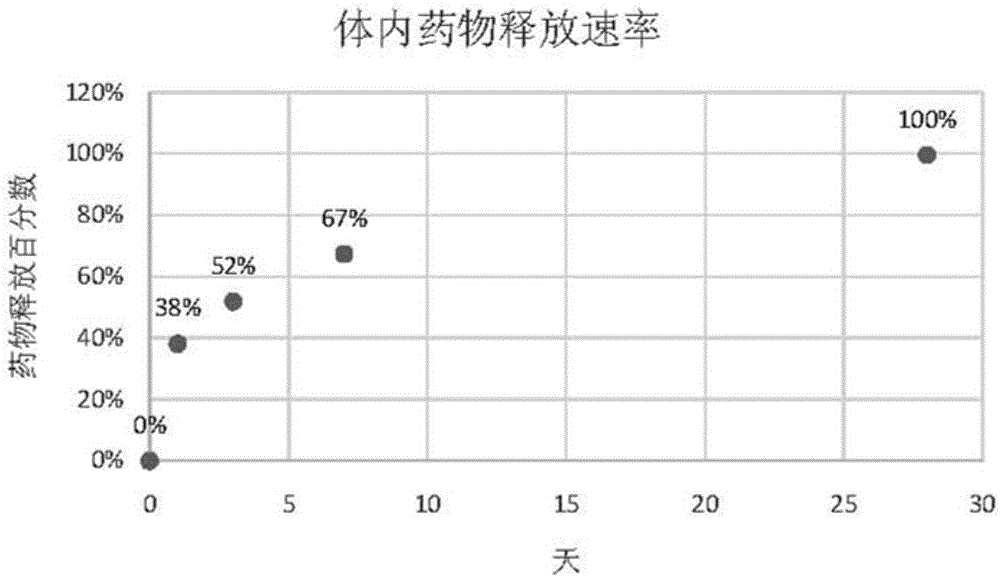
[0070] Embodiment 2 drug release kinetics test
[0071] The drug-eluting stent prepared in Example 1 was implanted in the iliofemoral artery of a rabbit (New Zealand albino rabbit), and the drug concentration of rapamycin in the blood of the rabbit, the concentration of the remaining drug in the stent in the blood vessel, and the concentration of the stent implanted in the artery were tested at different time points. Tissue drug concentration.
[0072] The stent was implanted in the rabbit after anesthesia, and heparin (1000IU / ml, 150IU / Kg) was injected after penetrating the 5F catheter sheath. Insert a 5F balloon catheter into the blood vessel through the catheter sheath to reach the iliac artery, expand it by 10-14 standard atmospheric pressure, and then send the test stent sample into the blood vessel, expand according to the ratio of the balloon size to the blood vessel size 1:1.3, and maintain the pressure After 30 seconds, the balloon delivery system was withdrawn, and ...
Embodiment 3
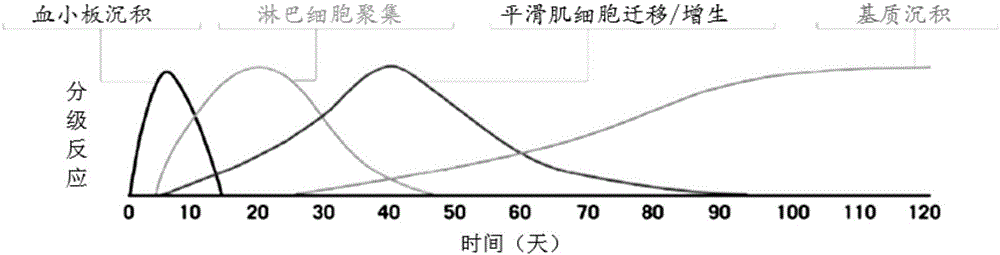
[0086] Example 3 Intracranial drug-eluting stent coating degradation and endothelial repair test
[0087] In a rabbit (New Zealand albino rabbit) iliofemoral artery model, a bare metal stent (Sino Medical) (BMS), a drug-eluting stent (IES) prepared in Example 1 of the present invention, and a base coating without a drug coating were implanted. Layer stent (BS) (its preparation method is identical with embodiment 1, just does not spray drug coating) and another kind of commercially available drug-coated stent (Cypher stent, U.S. Cordis company) (contrast), compare different stent endothelium coverage degree and endothelial activity. Endothelial activity is determined by CD-31 / PECAM-1 staining test, and mature endothelial cells will show a green fluorescent signal after staining.
[0088] The experimental results showed that after 90 days of implantation, both IES and BS achieved complete coverage of the endothelium, which was comparable to the BMS stent, and there was no drug ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com