Curcumin-amino acid conjugate and application
A technology of amino acids and conjugates, which is applied in the application field of curcumin-amino acid binary conjugates in the preparation of anti-inflammatory drugs, and can solve problems such as complex processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 curcumin
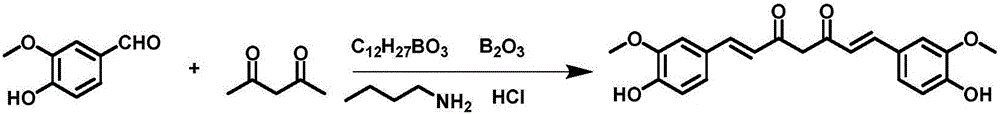
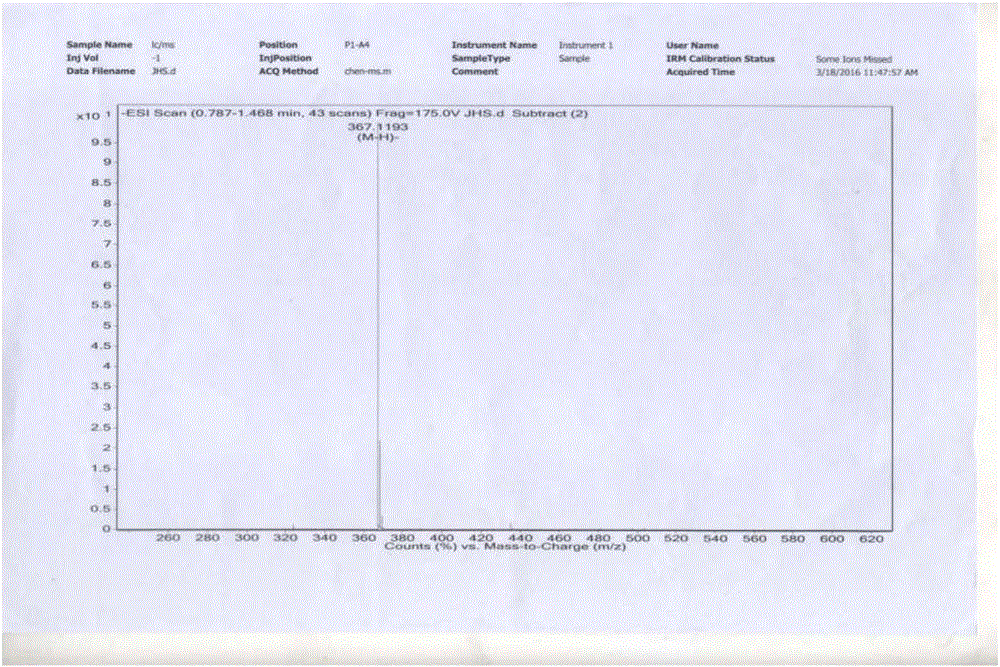
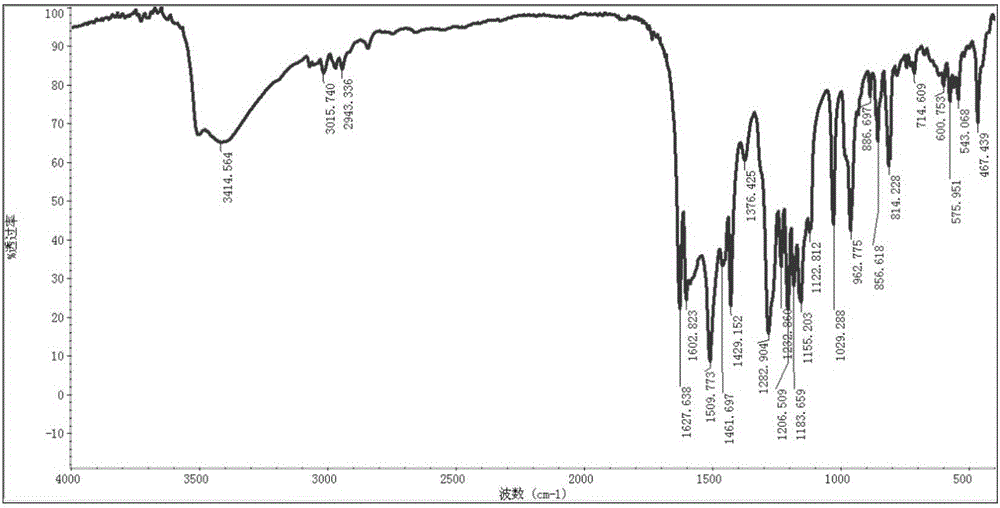
[0071] Take 60g of vanillin and 210mL of tributyl borate, add it to 200mL of dry ethyl acetate, stir and mix; then add 20g of acetylacetone and 10g of boric anhydride, stir at room temperature for 10min, then add dropwise 1-n-butylamine to catalyze the reaction, and stir at room temperature When the reaction is complete, add 300mL of dilute hydrochloric acid aqueous solution, stir at 60°C for 60min, separate the ethyl acetate layer, concentrate, add an appropriate amount of methanol to precipitate the product, leave it at -4°C, filter, and dry in vacuo to obtain 43.5g of yellow curcumin solids, Yield 62% (synthetic route sees figure 1 , see the characterization map Figure 2-4 ).
Embodiment 2
[0072] The preparation of embodiment 2 curcumin-glycine conjugates
[0073] Take 0.68g (9.0mmol) glycine and 0.76g sodium bicarbonate (9.0mmol), dissolve in 6mL deionized water, add 1.5mL aqueous formaldehyde (37%, 18.0mmol), stir at room temperature for 2 hours, then add 50mL methanol to dilute Above-mentioned reaction solution, then add 1.11g (3.0mmol) curcumin, stir reaction at room temperature for 2 hours, TLC monitors to curcumin reaction completely, add dropwise appropriate amount of 0.5M dilute hydrochloric acid to neutralize sodium bicarbonate, reaction product uses silica gel column chromatography Separation, dichloromethane / methanol / glacial acetic acid mixed solvent elution, obtain curcumin-glycine conjugate 0.89g, yield 43.2% (synthetic route sees Figure 5 ).
Embodiment 3
[0074] The preparation of embodiment 3 curcumin-alanine conjugates
[0075] Take 0.81g (9.0mmol) alanine and 0.76g sodium bicarbonate (9.0mmol), dissolve in 6mL deionized water, add 1.5mL formaldehyde aqueous solution (37%, 18.0mmol), stir at room temperature for 2 hours, then add 50mL Dilute the above-mentioned reaction solution with methanol, then add 1.11g (3.0mmol) curcumin, stir and react at room temperature for 3 hours, TLC monitors until the curcumin reaction is complete, add dropwise an appropriate amount of 0.5M dilute hydrochloric acid to neutralize sodium bicarbonate, and the reaction product is purified by silica gel column Chromatographic separation, dichloromethane / methanol / glacial acetic acid mixed solvent elution, to obtain curcumin-alanine conjugate 0.98g, yield 44.8% (synthetic route sees Image 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com