Polymorphic substances of obeticholic acid and preparation method thereof
A technology for obeticholic acid and polymorphs, which is applied in the field of polymorphs of obeticholic acid and its preparation, and can solve problems such as poor repeatability and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Preparation of obeticholic acid crystal form I:
[0021] Add 4.5g of obeticholic acid into 35ml of n-butyl acetate, heat up to reflux, stir for 2 hours, add 100ml of heptane, filter with suction when the temperature is lowered to room temperature, rinse the filter cake with heptane, and dry the filter cake by air blowing. 4.1 g of obeticholic acid in I crystal form was obtained, with a yield of 91% and a purity of 99%.
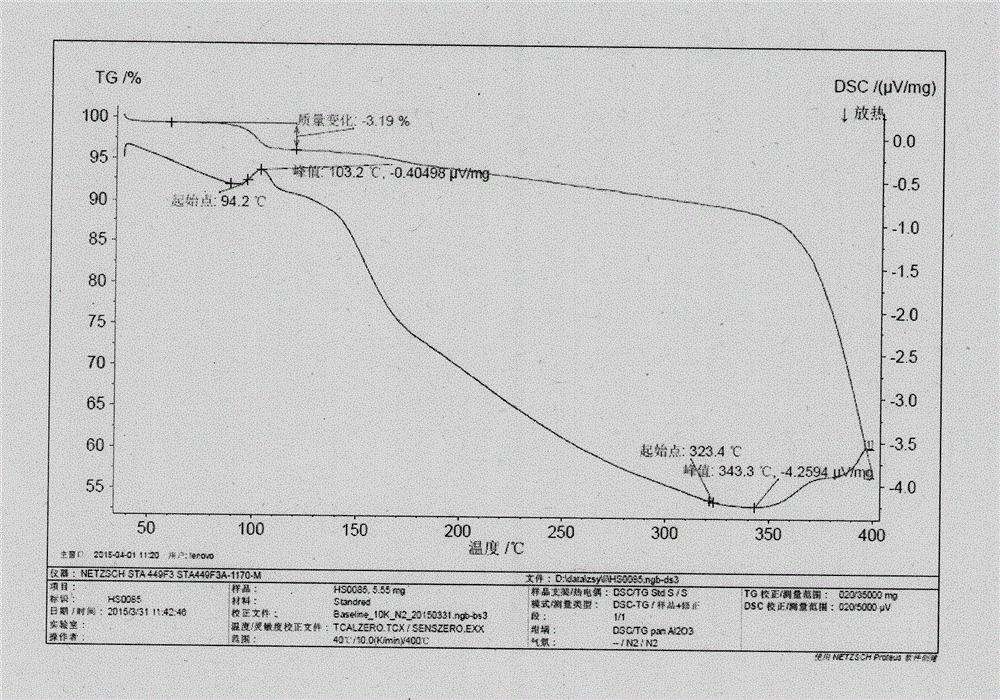
[0022] The DSC spectrum of crystal form I is as follows figure 1 As shown, there is an endothermic peak at 94±2.0°C.
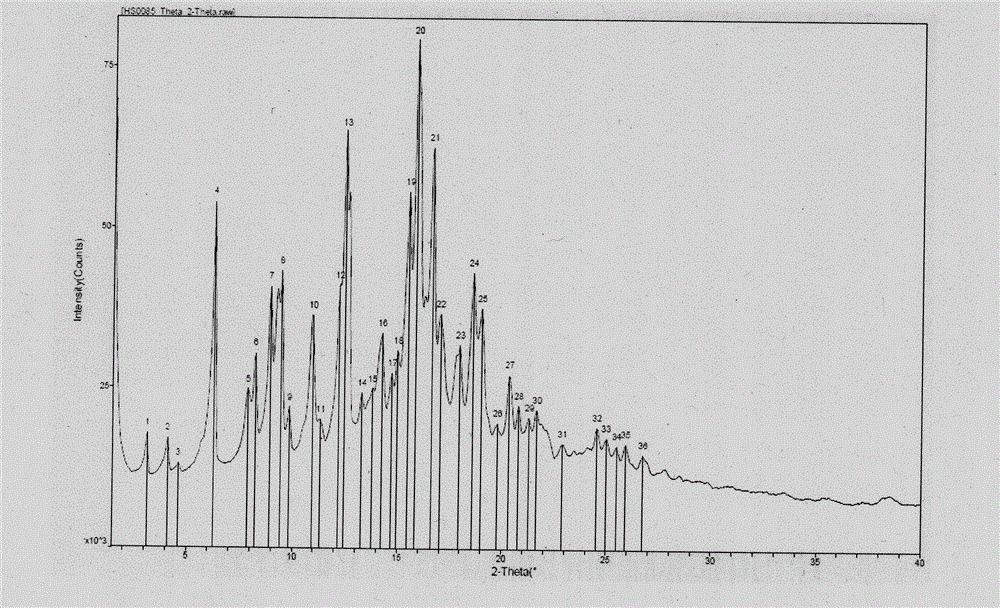
[0023] The XRPD spectrum of crystal form I (radiation source is CuKα1) is figure 1 As shown, there are diffraction peaks at 2θ values of 4.2, 6.3, 12.4, and 15.8, and the error range of 2θ values is ±0.2.
Embodiment 2
[0024] Embodiment 2 Preparation of obeticholic acid crystal form II:
[0025] Dissolve 30g of obeticholic acid in NaOH (8.3% NaOH, 45.0ml) solution, slowly add HCl (0.22mmol, 450ml) solution to the solution at 30-40°C, and adjust the pH to 2-5 , slowly cooled to room temperature, filtered the precipitate, and dried to obtain 28.4 g of crystal form II as a solid, with a yield of 94.6% and a purity of 99%.
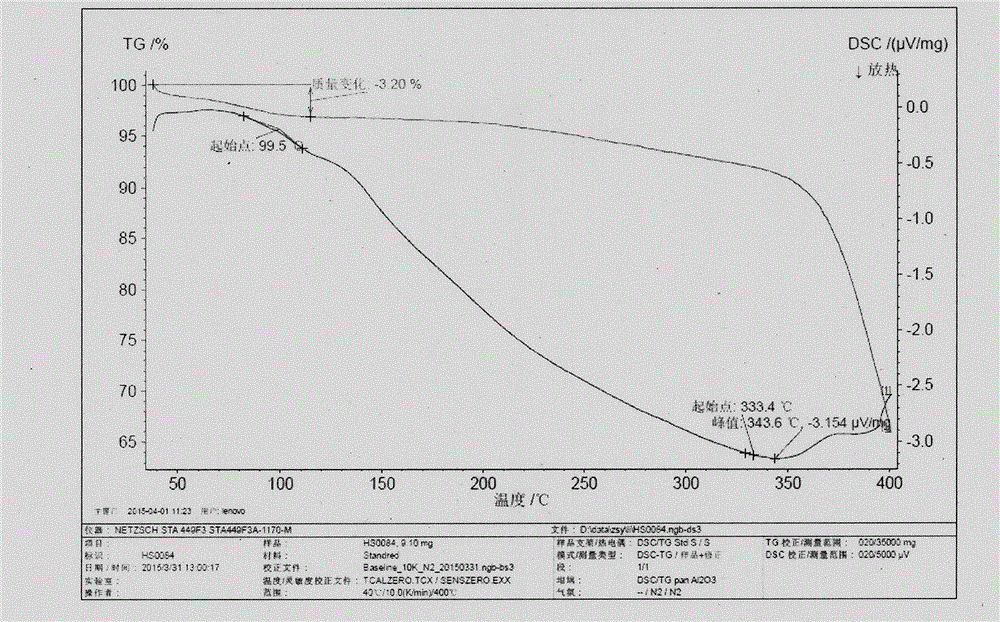
[0026] The DSC spectrum of crystal form II is as follows image 3 As shown, there is an endothermic peak at 99±2.0°C.
Embodiment 3
[0027] Embodiment 3 Preparation of obeticholic acid crystal form I:
[0028] Add 10g of obeticholic acid into 5ml of toluene, heat up to reflux, stir for half an hour, add 50ml of petroleum ether under reflux, stir for 2 hours, and suction filter when cooling down to room temperature, and rinse the filter cake with cyclohexane. The filter cake was blast-dried to obtain 9.3 g of obeticholic acid crystal form I with a yield of 93% and a purity of 99%.
[0029] The DSC spectrum of crystal form I is as follows figure 1 As shown, there is an endothermic peak at 94±2.0°C.
[0030] The XRPD spectrum of crystal form I (radiation source is CuKα1) is figure 2 As shown, there are diffraction peaks at 2θ values of 4.2, 6.3, 12.4, and 15.8, and the error range of 2θ values is ±0.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com