Method for preparing high-quality tilmicosin through low-quality tylosin
A technology of tylosin and tilmicosin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as non-compliance with the requirements of the pharmacopoeia, and achieve easy separation, recyclability, economical and environmental protection. Operation, the effect of saving the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
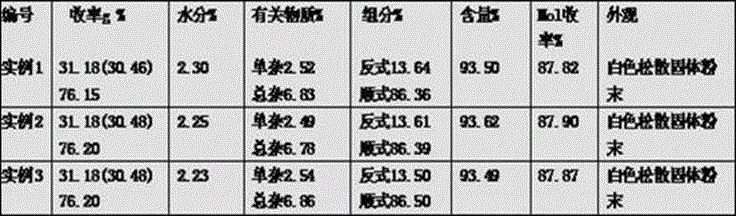
[0009] Example 1: Add 110ml of isoamyl acetate and 150ml of water to a 500ml reaction bottle and stir to dissolve 40g of tylosin tartrate. After complete dissolution, adjust the pH to 11 with 30% sodium hydroxide, stir for 10 minutes, and let stand to separate layers. Raise the temperature of the oil phase to 40°C, add 5.36g of 3,5-dimethylpiperidine at one time and stir, continue to heat up to 58°C, and slowly add the formic acid / isoamyl acetate mixture (2.52g formic acid, isoamyl acetate 13.34ml) was raised to 65°C for 4h, then lowered to 40°C. Add 150ml of water, adjust the pH to 2.5 with 15% hydrochloric acid, stir for 20 minutes and let stand to separate layers. The water phase is adjusted to PH=1.56. Maintain at 40°C for 60 minutes, and adjust the pH to 6 with 30% sodium hydroxide. Add 7.0g of Beta molecular sieves, stir at 45°C for 40min, and filter with suction. Slowly add 30% sodium hydroxide dropwise to the filtrate at 40°C to adjust the pH to 12, stir for 1h with...
example 2
[0010] Example 2: Add 115ml of isoamyl acetate and 160ml of water into a 500ml reaction bottle and stir to dissolve 40g of tylosin tartrate. After complete dissolution, adjust the pH to 11 with 30% sodium hydroxide, stir for 20 minutes, and let stand to separate layers. Raise the temperature of the oil phase to 40°C, add 5.37g of 3,5-dimethylpiperidine at one time and stir, continue to heat up to 58°C, and slowly add the formic acid / isoamyl acetate mixture (2.53g formic acid, isoamyl acetate 13.20ml) was raised to 65°C for 4.5h, then lowered to 40°C. Add 150ml of water, adjust the pH to 2.5 with 15% hydrochloric acid, stir for 20 minutes and let stand to separate layers. The water phase is adjusted to PH=1.56. Maintain at 40°C for 60 minutes, and adjust the pH to 6 with 30% sodium hydroxide. Add 7.5g Beta molecular sieves, stir at 47°C for 40min, filter with suction, slowly add 30% sodium hydroxide dropwise at 40°C to adjust the pH to 12, stir for 1h with suction, filter wi...
example 3
[0011] Example 3: Add 110ml of isoamyl acetate and 150ml of water into a 500ml reaction bottle and stir to dissolve 40g of tylosin tartrate. After complete dissolution, adjust the pH to 11 with 30% sodium hydroxide, stir for 10 minutes, and let stand to separate layers. Raise the temperature of the oil phase to 40°C, add 5.36g of 3,5-dimethylpiperidine at one time and stir, continue to heat up to 58°C, and slowly add the formic acid / isoamyl acetate mixture (2.52g formic acid, isoamyl acetate 13.30ml) was raised to 65°C for 5h, then lowered to 40°C. Add 160ml of water, adjust the pH to 2.5 with 15% hydrochloric acid, stir for 20 minutes and let stand to separate layers. The water phase is adjusted to PH=1.56. Maintain at 40°C for 60 minutes, and adjust the pH to 6 with 30% sodium hydroxide. Add 7.3g Beta molecular sieves, stir at 50°C for 40min, filter with suction, slowly add 30% sodium hydroxide dropwise to the filtrate at 40°C to adjust the pH to 12, stir for 1h with suct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com