Immunofluorescence kit for quantitatively detecting content of troponin I and preparation method
A troponin and immunofluorescence technology, which is applied in the field of quantitative detection of troponin I content immunofluorescence kits and preparation, can solve the problems of low sensitivity, inability to quantify, and poor repeatability, and achieve the effect of improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
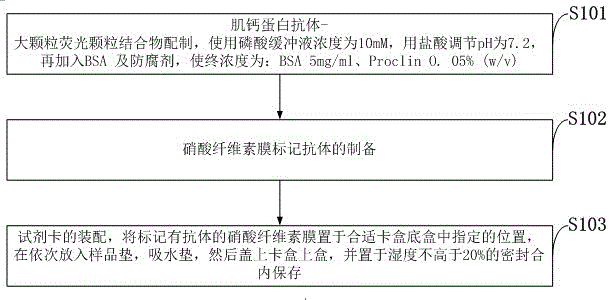
[0062] The preparation method of the antibody and particle complex comprises the following steps:
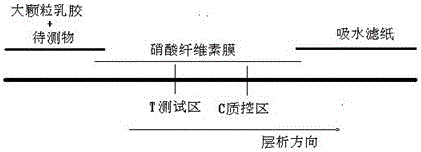
[0063] 1) Activation and washing of large particle latex particles, this step needs to activate the amino groups modified on the surface of large particles, centrifuge the commercialized latex latex solution after activation, discard the supernatant, and wash the precipitate repeatedly 3 times with washing buffer; The final precipitation is resuspended in the washing buffer, so that the mass volume ratio of the latex particles is 1% final concentration, and the washing buffer is selected from one of PBS buffer and MES buffer;
[0064] 2) Troponin I antibody coated latex particles
[0065] 1. Get 1ml of latex solution after activating and washing, add Troponin I antibody, make Troponin I antibody final concentration be 1mg / ml; After mixing, put 437 DEG C of shakers and coat with 220rpm rotating speed for 218h;
[0066] ②Wash the troponin I antibody not bound to the latex particl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com