Method for improving exocytosis level of recombinant protein of escherichia coli based on dacA
A technology of Escherichia coli and recombinant protein, applied in the fields of genetic engineering and fermentation engineering, can solve the problems of massive accumulation of intermediates, hindering protein production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 This example illustrates the effect of overexpression of dacA on the extracellular secretion of recombinant amylase by E.coli
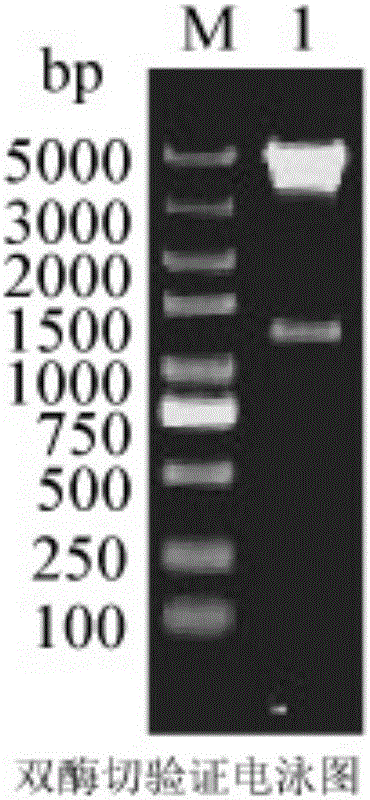
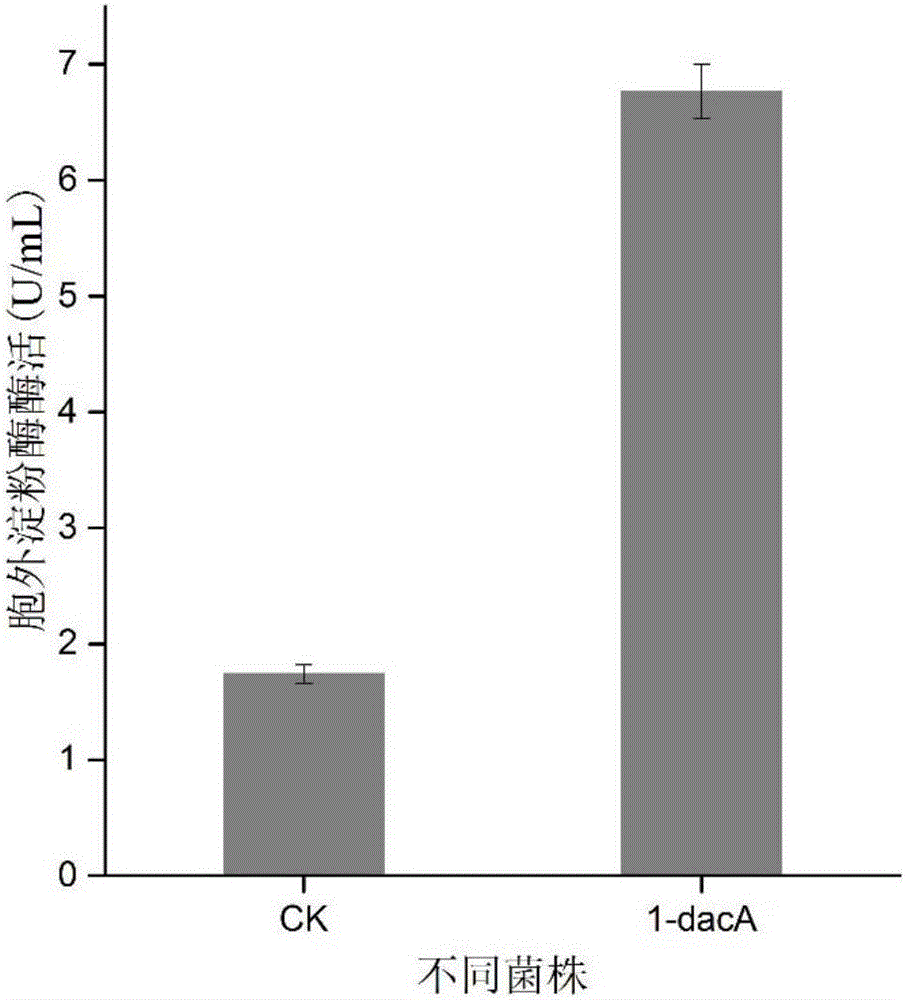
[0026] After double-enzyme digestion verification of the successfully constructed recombinant plasmid pRSFDuet-dacA ( figure 1 ), after being transformed into E.coliBL21(DE3) containing amylase recombinant plasmid pETDuet-AmyK, the level of extracellular secreted recombinant amylase in E.coli overexpressing carboxypeptidase dacA was significantly increased when the fermentation was induced for 24 hours, and the extracellular starch Enzyme activity reached 6.77U / mL ( figure 2 ). However, after induction and fermentation of the control strain without overexpressing any carboxypeptidase for 24 hours, the enzyme activity of the recombinant amylase measured outside the E.coli cell was only 1.74U / mL.
Embodiment 2
[0027] Example 2 This example illustrates the effect of overexpression of dacA on the distribution of recombinant amylase in E.coli cells
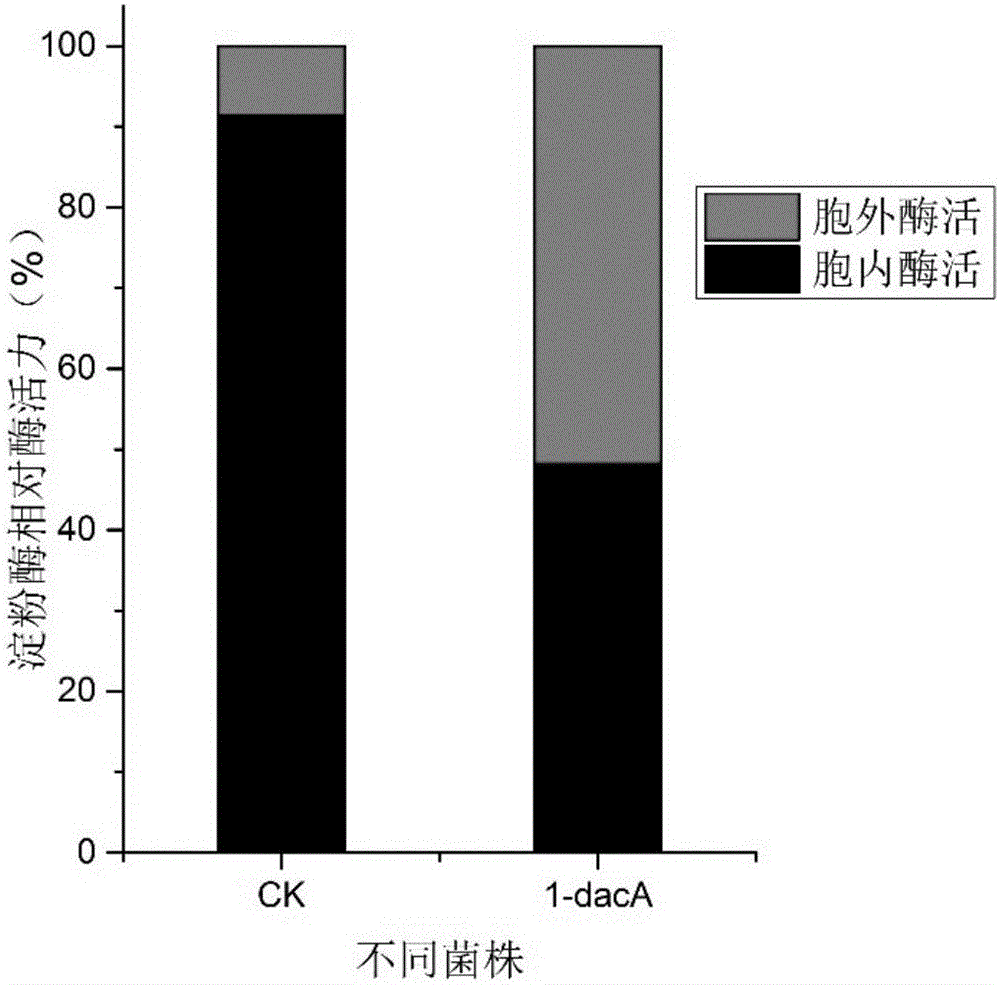
[0028] When carboxypeptidase dacA was overexpressed in E.coli, the intracellular content of recombinant amylase decreased from 91.46% of the control group to 48.23% after induction for 24 hours. The content of extracellular recombinant amylase was increased from 8.54% of the control group to 51.77% ( image 3 ).
Embodiment 3
[0029] Example 3 This example illustrates the effect of overexpression of dacA on soluble peptidoglycan in E.coli cells
[0030] When the carboxypeptidase dacA was overexpressed in E.coli, the content of soluble peptidoglycan in E.coli cells was significantly increased after induction for 6 hours, and the content of intracellular soluble peptidoglycan reached 92.79 mg / g ( Figure 4 ). However, the content of soluble peptidoglycan in E.coli cells was only 29.17mg / g after induction and fermentation of the control strain without any carboxypeptidase overexpression for 6h.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com