Preparation method of canagliflozin intermediate
A technology of Grignard reagent and methyl group, which is applied in the field of drug synthesis and can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
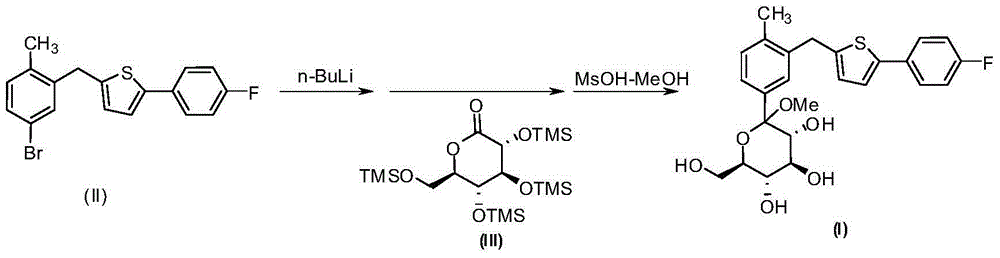
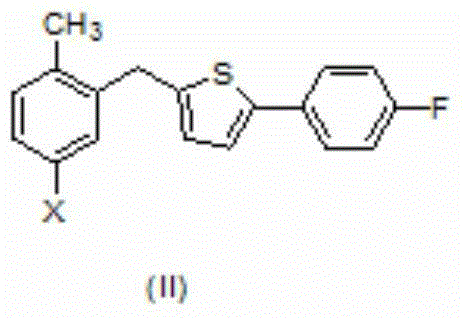
[0026] Magnesium powder (14.4g, 600mmol) and iodine (1.3g, 10.0mmol) were added to a dry reaction flask, dry tetrahydrofuran (50mL) was added to the reaction flask under nitrogen protection, cooled to 0°C, and 2- [(5-bromo-2-methylphenyl)methyl]-5-(4-fluorophenyl)thiophene (II) (36.1g100mmol) in tetrahydrofuran (100mL) solution was slowly added dropwise to the reaction flask, and the addition was completed Raise the temperature to 25°C, stir the reaction for 5-8 hours, cool down to 0°C, add the above reaction solution to 2,3,4,6-tetra-O-trimethylsilyl-D-glucose dropwise at 0°C Acid lactone (III) (55.9g, 120mmol) in anhydrous tetrahydrofuran (100mL) solution, the reaction mixture was stirred for 2 ~ 3h after the addition was completed, and the mixture containing methanesulfonic acid (26.4g, 275mmol) The methanol (150mL) solution was added dropwise to the above reaction solution, and the temperature was slowly raised to room temperature and stirred overnight. After the reaction ...
Embodiment 2
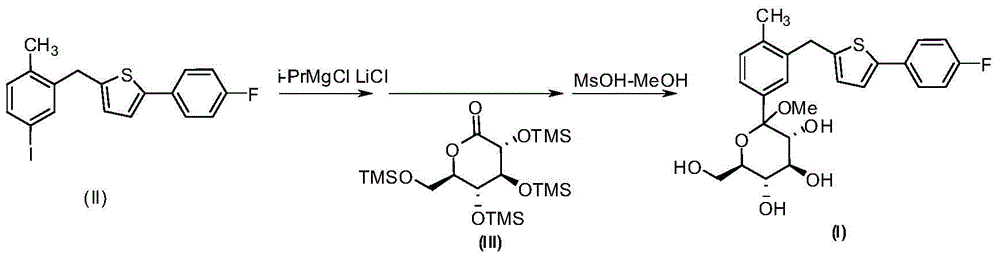
[0029] Magnesium powder (14.4g, 600mmol) was added to a dry reaction flask, dry tetrahydrofuran (50mL) was added to the reaction flask under nitrogen protection, cooled to 0°C, and 2-[(5-iodo-2-methyl Phenylphenyl)methyl]-5-(4-fluorophenyl)thiophene (II) (41.0g100mmol) in tetrahydrofuran (100mL) was slowly added dropwise to the reaction flask, and the temperature was raised to 25°C after the addition, and the reaction was stirred for 5~ 8h, cool down to 0°C, add the above reaction solution dropwise to 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone (III) (55.9g , 120mmol) in anhydrous tetrahydrofuran (100mL) solution, after addition, the reaction mixture was stirred for 2-3h, and was added dropwise to methanol (150mL) solution containing methanesulfonic acid (26.4g, 275mmol) under cooling condition In the above reaction solution, slowly warm up to room temperature and stir overnight after dropping. After the reaction, lower the temperature to 0°C and add about 300mL saturated ...
Embodiment 3
[0031]Magnesium powder (14.4g, 600mmol) and iodine (1.3g, 1.0mmol) were added to a dry reaction flask, and dry ether (50mL) was added to the reaction flask under nitrogen protection, cooled to 0°C, and 2- [(5-Bromo-2-methylphenyl)methyl]-5-(4-fluorophenyl)thiophene (II) (36.1g, 100mmol) in ether (100mL) solution was slowly added dropwise to the reaction flask, After the addition, the temperature was raised to 25°C, stirred for 5-8 hours, then cooled to 0°C, and the above reaction solution was added dropwise to 2,3,4,6-tetra-O-trimethylsilyl-D at 0°C -In a solution of gluconolactone (III) (55.9g, 120mmol) in anhydrous tetrahydrofuran (100mL), the reaction mixture was stirred for 2-3h after the addition was completed, and the mixture containing methanesulfonic acid (26.4g, 275mmol) of methanol (150mL) solution was added dropwise to the above reaction solution, and after the dropping, the temperature was slowly raised to room temperature and stirred overnight. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com