Method for preparing nitrogen-doped graphene material in electrochemical stripping mode
A nitrogen-doped graphene, electrochemical technology, applied in chemical instruments and methods, graphene, inorganic chemistry, etc., can solve the problems of poor dispersion performance, toxic precursors, poor oxygen reduction activity, etc., to achieve high current density, Good water dispersibility and good methanol resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve 0.56g of sodium sulfate, 2.5g of melamine, and 7.2mL of formaldehyde in 40mL of deionized water, adjust the pH of the solution to 6.0-9.0, use a graphite sheet as the positive electrode and a stainless steel sheet as the negative electrode, and apply 10V DC. After the expansion is completed, it is taken out and dried, and then calcined in a tube furnace at 650°C for 3 hours to obtain nitrogen-doped expanded graphite;
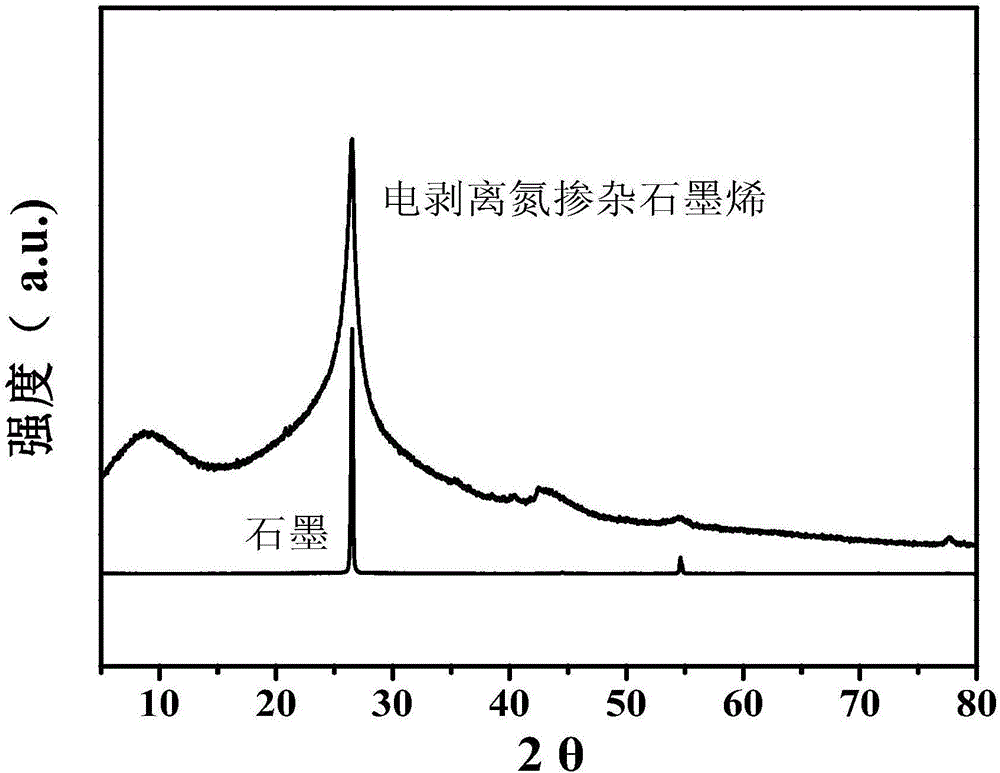
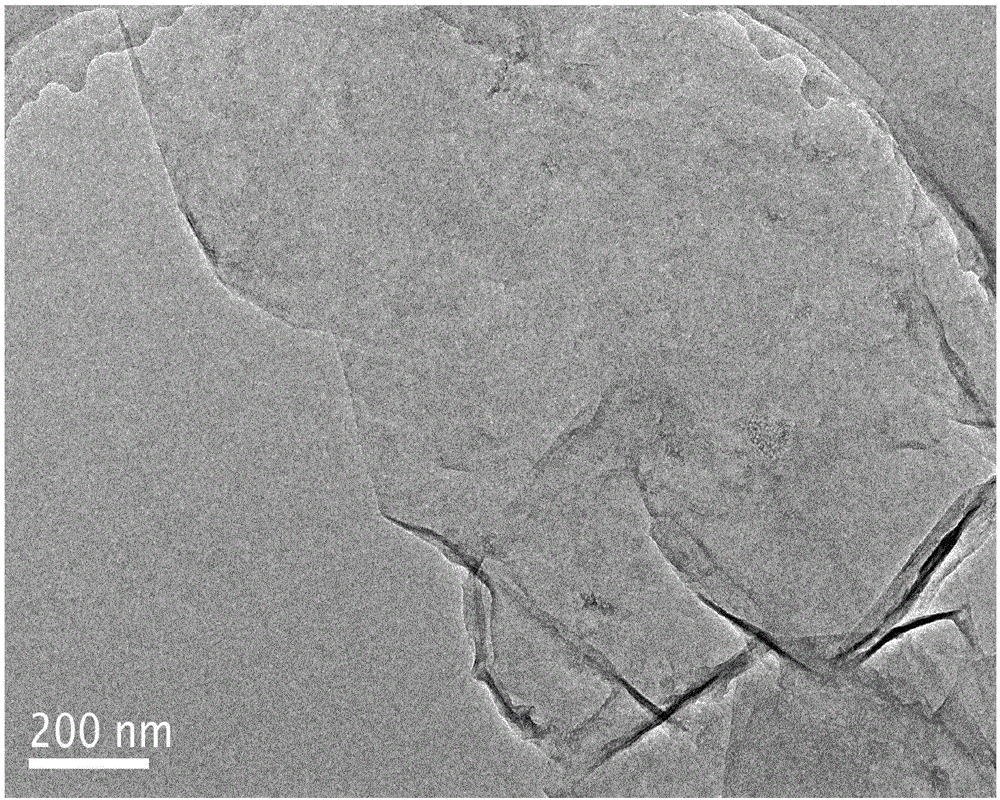
[0024] (2) Stripping the nitrogen-doped expanded graphite prepared in step (1) in a 0.5M ammonium sulfate solution with pH=6.0 to 9.0 by 10V direct current, suction filtering, washing and drying the stripped product to obtain the product. After XRD identification and SEM and TEM techniques, the product is electroexfoliated nitrogen-doped graphene.
Embodiment 2
[0026] (1) Dissolve 2.84g of sodium sulfate, 2.5g of dicyandiamide, and 3.6mL of formaldehyde in 40mL of deionized water, adjust the pH of the solution to 6.0-9.0, use a graphite sheet as the positive electrode and a stainless steel sheet as the negative electrode, and apply 2V direct current. After the expansion is completed, it is taken out and dried, and then calcined in a tube furnace at 750°C for 2 hours to obtain nitrogen-doped expanded graphite;
[0027] (2) Stripping the nitrogen-doped expanded graphite prepared in step (1) in a 0.1M ammonium sulfate solution with pH=6.0 to 9.0 by 10V direct current, suction filtering, washing and drying the stripped product to obtain the product. After XRD identification and SEM and TEM techniques, the product is electroexfoliated nitrogen-doped graphene. .
Embodiment 3
[0029] (1) Dissolve 5.6g of sodium sulfate, 2.5g of dicyandiamide, and 25.2mL of formaldehyde in 40mL of deionized water, adjust the pH of the solution to 6.0 to 9.0, use a graphite sheet as the positive electrode and a stainless steel sheet as the negative electrode, and apply 10V direct current. After the expansion is completed, it is taken out and dried, and then calcined in a tube furnace at 550°C for 6 hours to obtain nitrogen-doped expanded graphite;
[0030] (2) Stripping the nitrogen-doped expanded graphite prepared in step (1) in a 0.1M ammonium sulfate solution with a pH of 6.0 to 9.0 through 15V direct current, and suction filtering, washing and drying the stripped product to obtain the product. After XRD identification and SEM and TEM techniques, the product is electroexfoliated nitrogen-doped graphene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com