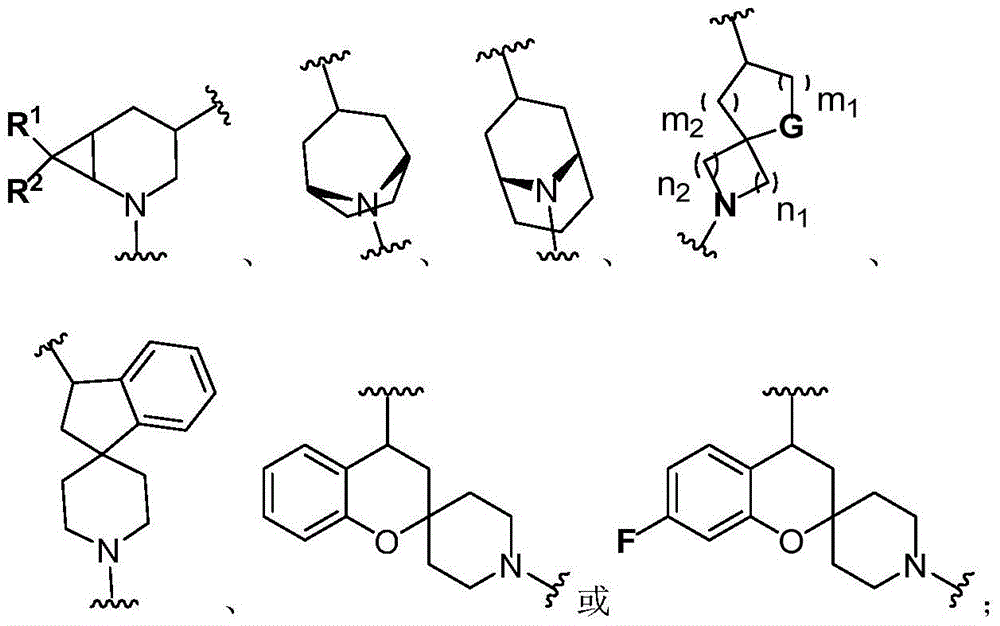
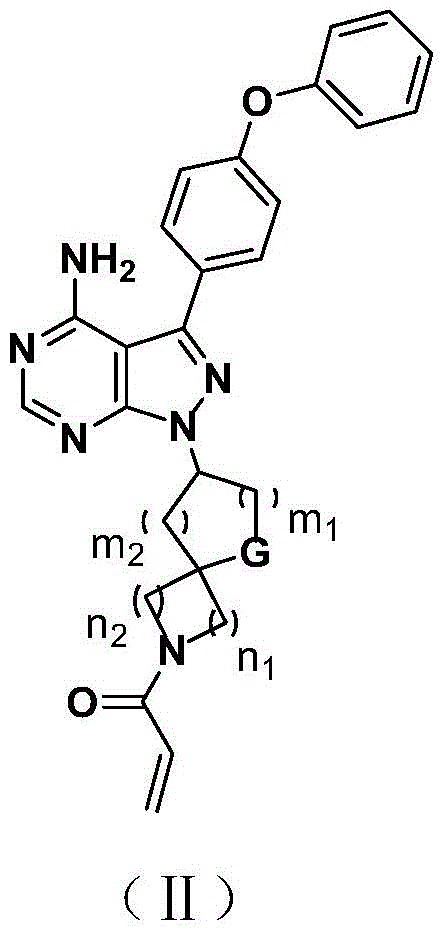
Bruton tyrosine kinase inhibitor with spiro or bridge ring structure and preparation method thereof
A technology of tyrosine kinase and bridged ring structure, applied in the field of medicinal chemistry, can solve the problems of large clinical dosage, easy to be metabolized, low bioavailability, etc., and achieve the effect of strong inhibitory activity and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: 1-(3-(amino-3-(4-phenoxyphenyl)-3a,7a-1H-pyrazol[3,4-d]pyrimidin-1-yl)-2,3- Preparation of dihydrospiro[indene-1,4'-piperidin]-1'-yl)prop-2-en-1-one,
[0039] The synthesis steps are as follows:
[0040]
[0041] Step 1: Preparation of N,N-bis(2-chloroethyl)carbamate tert-butyl ester (1b)
[0042] Under ice-bath condition, add two (2-chloroethyl) amine hydrochloride (19.99g, 0.112mmol) and (Boc) 2 Slowly add TEA (34.00g, 0.336mmol) into 300mL DCM solution of O (26.84g, 0.123mmol), wait for the reaction mixture to naturally rise to room temperature and continue to stir for 5h, TLC shows that after the reaction of raw materials is completed, the reaction solution is saturated with water and washed with brine, and the organic phase was washed with anhydrous Na 2 SO 4 Fully dried, evaporated in vacuo and purified by flash chromatography (PE:EA=100:1) to obtain 24.4 g of the target compound as a colorless oil;
[0043] Step 2: Preparation of spiro[indene-1...
Embodiment 2
[0057] Endo-1-((1R,3s,5S)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-1-yl) -8-Azabridge[3.2.1]octane-8-yl)prop-2-en-1-one and exo-1-((1R,3s,5S)-3-(4-amino-3 -(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-1-yl)-8-azabridge[3.2.1]octane-8-yl)prop-2- Preparation of en-1-ones
[0058]
[0059] Step 1: Preparation of N-tert-butoxycarbonyl-nortropinone
[0060]
[0061] To a solution of nortropinone hydrochloride (4.8 g, 29.70 mmol) and TEA (4.50 g, 44.55 mmol) in DCM (150 mL) was slowly added (Boc) under nitrogen. 2 O (56mg, 44.55mmol), the reaction mixture was stirred at room temperature overnight, after the reaction was completed, water (50mL) was added to quench the reaction, the organic phase was separated, and the aqueous phase was extracted with DCM (30mLx3), and the above organic phases were combined for Anhydrous Na 2 SO 4 Fully dried, evaporated in vacuo and purified by chromatography (PE:EA=5:1~1:1) to obtain 5.0 g of the target compound as a whit...
Embodiment 3
[0090] 1-((1R,5S)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidin-1-yl)-9-aza Preparation of bridge [3.3.1] nonan-9-yl) prop-2-en-1-one
[0091] The synthesis steps are as follows:
[0092]
[0093] Step 1: Preparation of 9-benzyl-9-azabicyclo[3.3.1]nonan-3-one
[0094] Add 1,3-acetonedicarboxylic acid to 20 mL of water containing benzylamine hydrochloride (3.8 g, 26.46 mmol), glutaraldehyde (25% aqueous glutaraldehyde, 8.8 mL, 21.90 mmol) under ice bath (3.2g, 21.90mmol) and 10% sodium acetate (7.5mL), after which the ice bath was removed, the reaction mixture was stirred at room temperature for 2h, then at 50°C for 12h, then solid NaHCO was added slowly 3 When the pH was greater than 7, the organic phase was separated, the aqueous phase was extracted with DCM (20mLx3), and the above organic phases were combined with anhydrous Na 2 SO 4 Fully dried, evaporated in vacuo and purified by chromatography (PE:EA=10:1) to obtain 2.3 g of the target compound as a whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com