Polyoxometalate compound modified by amino acid and preparation method and application of polyoxometalate compound
A technology of heteropoly acid salts and amino acids, which can be used in drug combination, organic chemistry, antineoplastic drugs, etc., and can solve the problem of low anti-leukemia activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1K2
[0030] Example 1K 2 Na[AsMo 6 o 21 (O 2 CCH 2 NH 3 ) 3 ]·6H 2 Synthesis of O
[0031] Na 2 MoO 4 ·H 2 O (6.0mmol), As 2 o 3 (1.1mmol), glycine (6.0mmol), KCl (6.0mmol) dissolved in H 2 O, placed on a magnetic stirrer for mixing and stirring, adjusted the pH of the solution to 3.50 with HCl, stirred and heated to reflux for 1 h. Let the solution stand until it is completely cooled, filter it into a 100ml small beaker, and let it stand for 1-2 days to form yellow crystals with a yield of 89%.
Embodiment 2
[0032] Example 2K 2 Na[AsMo 6 o 21 (O 2 CCH 2 NH 3 ) 3 ]·6H2 Structural identification of O
[0033] (1) Compound (AsK 2 NaMo 6 o 33 C 6 h 27 N 3 ) by infrared spectroscopy detection
[0034] Use AlphaCentauriFT / IR infrared spectrometer, press with KBr, at 400-4000cm -1 The IR spectra of the compounds were determined in the range of . IR(KBr):3536,3157,2708,2639,1611,1496,1462,1416,1332,1115,1052,905,778,652,556,425cm -1 。
[0035] (2) Compound (AsK 2 NaMo 6 o 33 C 6 h 27 N 3 ) UV Spectroscopic Detection
[0036] The compound was dissolved in water, and the absorption peak of the compound was measured using a UV-1800 ultraviolet-visible absorption spectrometer at a wavelength of 190-400 nm. UV spectrum see figure 1 , UV: 207nm, 226nm.
[0037] (3) compound (AsK 2 NaMo 6 o 33 C 6 h 27 N 3 ) elemental analysis
[0038] The compound (AsK 2 NaMo 6 o 33 C 6 h 27 N 3 ) for elemental analysis of C, H and N; the compound (AsK 2 NaMo 6 o 33 C ...
Embodiment 3
[0041] Embodiment 3MTT method measures the antitumor activity and cytotoxicity of test substance
[0042] (1) Main experimental materials
[0043] Cell lines: HL60, U937 and HUVEC cells were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences.
[0044] (2) Determination of the antitumor activity of the test substance
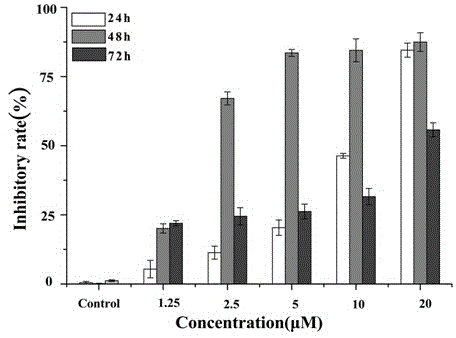
[0045] HL-60 and U937 cells were cultured to the logarithmic growth phase, and then 1.0×10 4 The density of cells / mL was inoculated on a 96-well plate, and different concentration gradients of the test substance (1.25, 2.5, 5, 10, 20 μM) were added, and the medium without drugs was added to the control wells, and duplicate holes were set. The same volume of culture solution was given to the negative control group. After culturing for 24, 48 and 72 hours in a 5% CO2, 37°C incubator, add MTT in CO 2 After culturing in the incubator for 4 hours, add 100 μL triple solution (10% SDS, 5% isopropanol, 12 mmol / L H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com