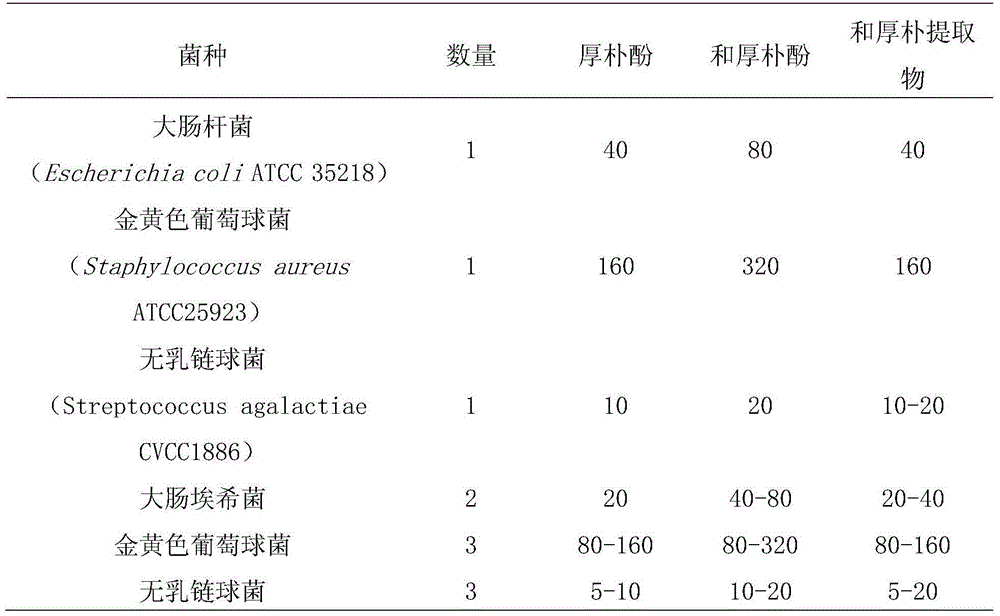
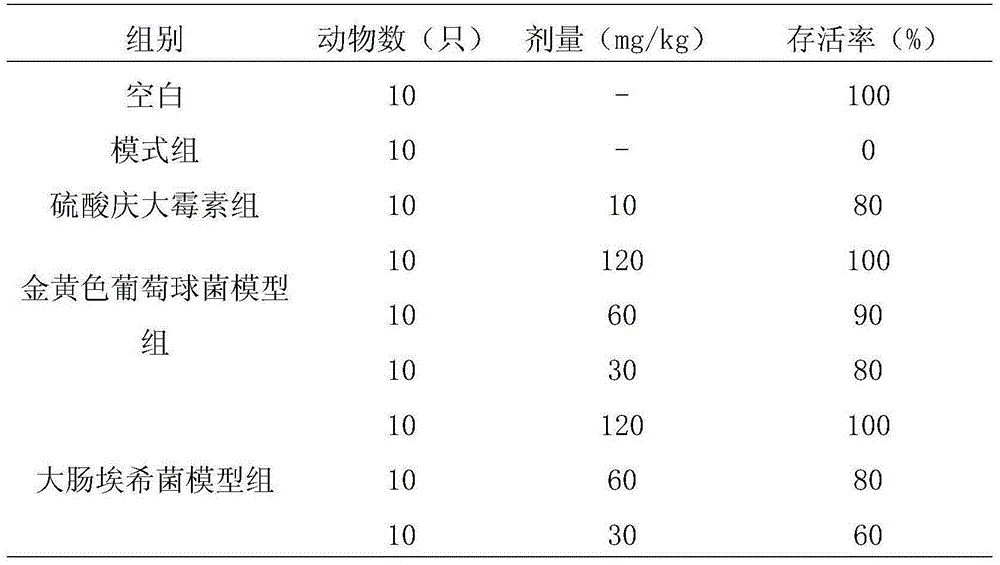
Application of officinal magnolia bark extract to prepare medicine for treating cow mastitis
A cow mastitis, extract technology, applied in the field of natural compounds, can solve the problem of preventing and treating cow mastitis with magnolia bark extract, and achieve the effect of low toxicity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The preparation of embodiment 1 Magnolia officinalis extract
[0017] Using Magnolia officinalis as a raw material, crush the Magnolia officinalis, extract with n-hexane 6 times the weight of Magnolia officinalis powder at 55°C-60°C, extract 3 times, extract for 2 hours each time, filter, and extract 3 times The filtrates were combined and concentrated under reduced pressure to form an extract. Add 25% of the paste weight of silica gel into the extract, stir evenly, and then dry it in vacuum, add cyclohexane 5 times the weight of the vacuum dry powder and extract in a water bath at 80°C, extract 3 times, each time for 30min, and extract the cyclohexane extract Concentrate to 1 / 5 of the original volume, filter the crude crystals with suction, dry the crude crystals to remove residual cyclohexane, then add petroleum ether 40 times the weight of the crude crystals, heat in a water bath at 70°C until the supernatant is clear, pour Go out supernatant liquid, let cool and pr...
Embodiment 2
[0018] The separation of embodiment 2 magnolol and honokiol
[0019] According to the method of the patent "a method for extracting honokiol and magnolol from magnolia crude extract" (patent application publication number: CN103922899A), magnolol and magnolol in the magnolo extract in Example 1 are separated. Honokiol. The specific method is as follows: take 1 kg of Magnolia officinalis extract in Example 1, add 10 L of cyclohexane, heat and reflux at 80 ° C in a 100 L reaction kettle for 3 h; take another 100 kg of purified water, put it in an open pot, add it to 80 ° C, and add hydrogen Sodium oxide 90g, sodium bicarbonate 210g, stirred to fully dissolve into a buffer solution with a pH of 10.5. Turn on the reactor mixer, add 50kg of buffer solution while stirring, and then stir for 15 minutes, then let stand and separate layers. Operate in the same way, extract five times in total, take a sample of the residual liquid to detect no magnolol spots, take purified water to pr...
Embodiment 3
[0020] The preparation of embodiment 3 magnolia officinalis extract emulsion
[0021] Take by weighing Magnolia officinalis extract 5g, propylene glycol 10g, soybean oil 30g, vitamin E 0.01g in the embodiment 1, stir continuously 10min to dissolve under the water-bath ultrasonic condition, obtain oily phase; Take soybean lecithin 2g, add water for injection appropriate amount , stirred and dissolved in a water bath to obtain a water phase; under the condition of stirring at 70°C, slowly inject the oil phase into the water phase, add water for injection to make the volume to 100mL, and obtain colostrum. Then the colostrum was passed through a colloid mill for 20 minutes, and finally divided, filled with nitrogen, and sterilized at 121°C for 15 minutes to obtain the obtained product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com