Process for obtaining heat sterilizable injectable hydrogels based on hyaluronic acid containing lidocaine and alkaline agents added in powder form
A technology of hyaluronic acid and lidocaine, applied in the field of therapy and cosmetology, which can solve the problems of injectable hydrogels changing flow properties and methods not fully satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
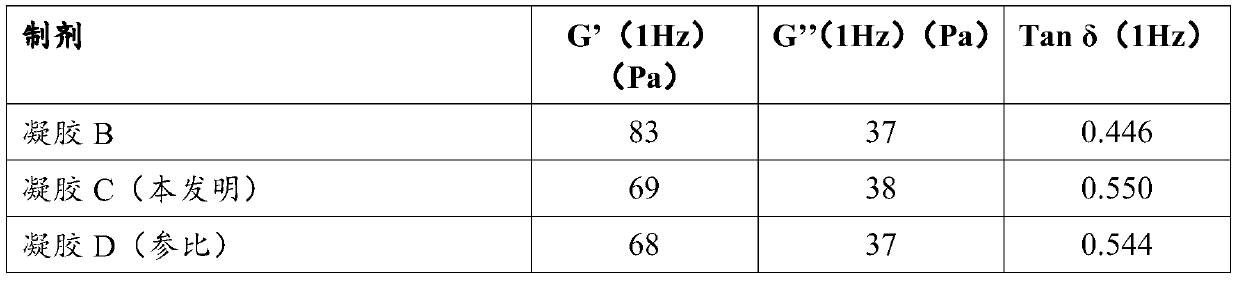
[0071] Example 1: Structural differences after moist heat sterilization demonstrated by rheology between hyaluronic acid-based gels with and without lidocaine hydrochloride and with lidocaine hydrochloride and sodium hydroxide
[0072] Let the cross-linked NaHA based gel be A.
[0073] This gel is obtained as follows: Molecular mass 2.5 x 10 hydrated in 1 mass % aqueous sodium hydroxide soda solution 6 Da of sodium hyaluronate, crosslinking agent BDDE added to obtain a degree of crosslinking of 9%, crosslinking for 2 h at 50° C. and dialyzing the gel for 24 h (regenerated cellulose, separation limit: molar mass = 60 kDa). The gel thus obtained had a sodium hyaluronate concentration of 22.5 mg / ml and a pH of 7.07.
[0074] After mixing with a spatula for 10 minutes, the gel A thus obtained was divided into three portions (50 g) of equal mass.
[0075] Let part 1 be B. In this part, 0.3% lidocaine hydrochloride is added as a powder. The gel was mixed with a spatula for 5 m...
Embodiment 2
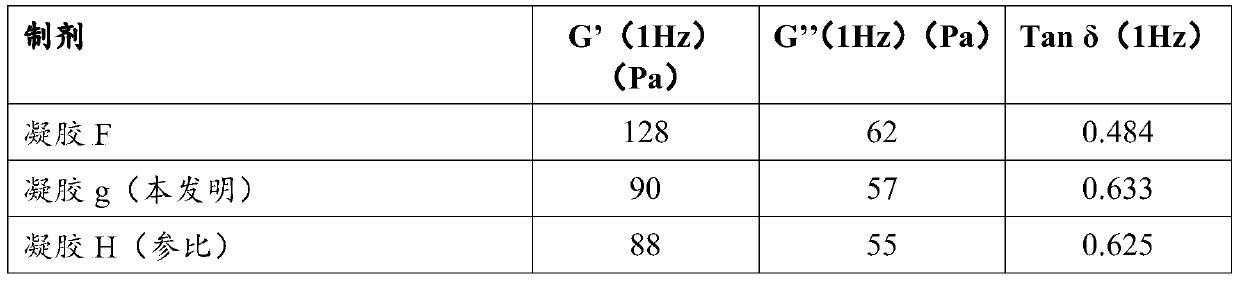
[0087] Example 2: Structural differences after moist heat sterilization demonstrated by rheology between hyaluronic acid-based gels with and without lidocaine hydrochloride and with lidocaine hydrochloride and sodium hydroxide
[0088] Let the cross-linked NaHA based gel be E.
[0089] This gel is obtained as follows: Molecular mass 2.5 x 10 hydrated in 1 mass % sodium hydroxide solution 6Da of sodium hyaluronate, crosslinking agent BDDE added to obtain a degree of crosslinking of 11%, crosslinking for 2 h at 50° C. and dialyzing the gel for 24 h (regenerated cellulose, separation limit: molar mass = 60 kDa). The gel thus obtained had a sodium hyaluronate concentration of 22.7 mg / ml and a pH of 7.12.
[0090] After mixing with a spatula for 10 minutes, the gel E thus obtained was divided into three portions (50 g) of equal mass.
[0091] Let part 1 be F. In this part, 0.3% lidocaine hydrochloride is added as a powder. The gel was mixed with a spatula for 5 minutes. Then...
Embodiment 5
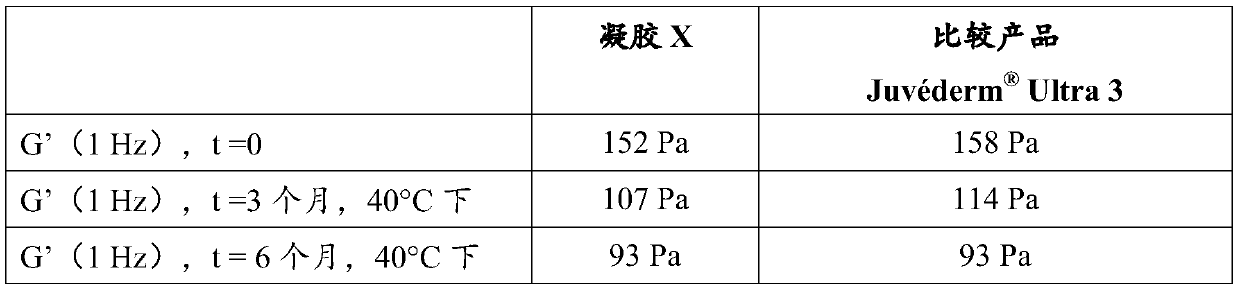
[0112] Example 5: Stability of the gels of the present invention - comparison with prior art stable gels
[0113] The cross-linked NaHA-based gel was prepared as follows: Hydration molecular mass 3.1 × 10 in 1 mass % aqueous sodium hydroxide solution 6 Da of sodium hyaluronate, crosslinking agent BDDE added to obtain a degree of crosslinking of 9.2%, crosslinking for 2 hours at 50 and dialysis for 24 hours (regenerated cellulose, separation limit: molar mass = 60 kDa). The gel thus obtained had a sodium hyaluronate concentration of 24.9 mg / ml and a pH of 7.08.
[0114] Next 0.3% lidocaine hydrochloride was added and the gel was mixed with a spatula for 5 minutes. The pH was found to be 6.77.
[0115] Finally, 144 μl of a 2 mass % aqueous sodium hydroxide solution was added and mixed with a spatula for 5 minutes. The final pH was 7.26.
[0116] The thus prepared gel was introduced into a glass syringe and then heat sterilized (131°C, 2 min).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com