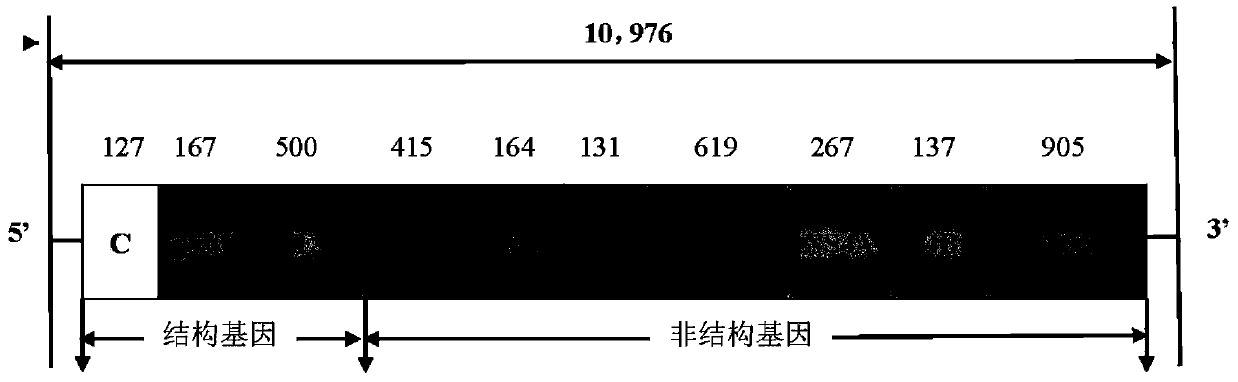
Live attenuated Japanese encephalitis vaccine strain sa14-14-2 adapted to human diploid cell 2bs and its vaccine
A technology of human diploid cells and live attenuated vaccines, applied in the field of virus vaccines, can solve the problems of carcinogenesis, increased vaccine cost, contamination by exogenous factors, etc., and achieves good tolerance, no contamination by exogenous factors, and high immunity original effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The present invention will be further described through specific embodiments below in conjunction with the accompanying drawings, but the protection scope of the present invention is not limited in any way.
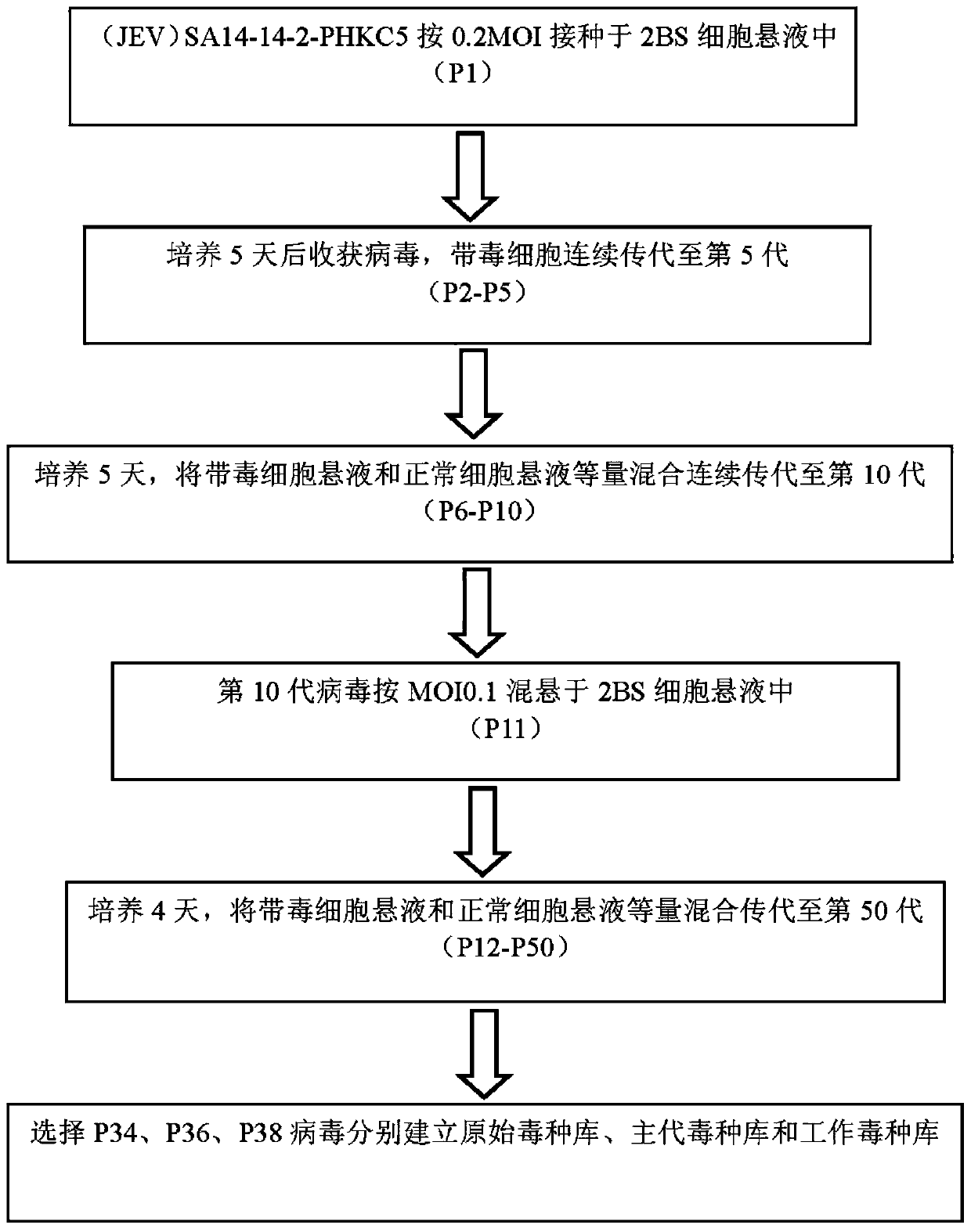
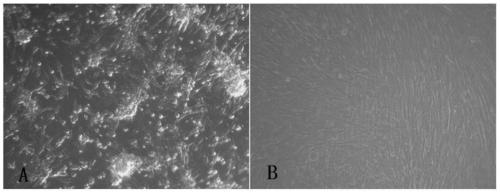
[0038] 1. Adaptation and passage of JEV (Japanese encephalitis attenuated vaccine strain SA14-14-2) on 2BS cells
[0039] 1. Source of cells and viruses
[0040] The cells used in this work are 2BS cells (27-37 passages), which were provided by the Cell Collection Center of China National Institutes for Food and Drug Control. The cell culture medium was Eagle's MEM containing 10% fetal bovine serum. The JE attenuated vaccine strain SA14-14-2 virus is the Japanese encephalitis virus (JEV) SA14-14-2-PHKC5 preserved in our laboratory.
[0041] 2. Adaptation and passage of SA14-14-2 on 2BS cells
[0042] (1) Digest the 2BS cells that have formed a monolayer with 0.25% trypsin, resuspend the cells with Eagle's MEM-10% fetal calf serum culture medium, inoculate the vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com