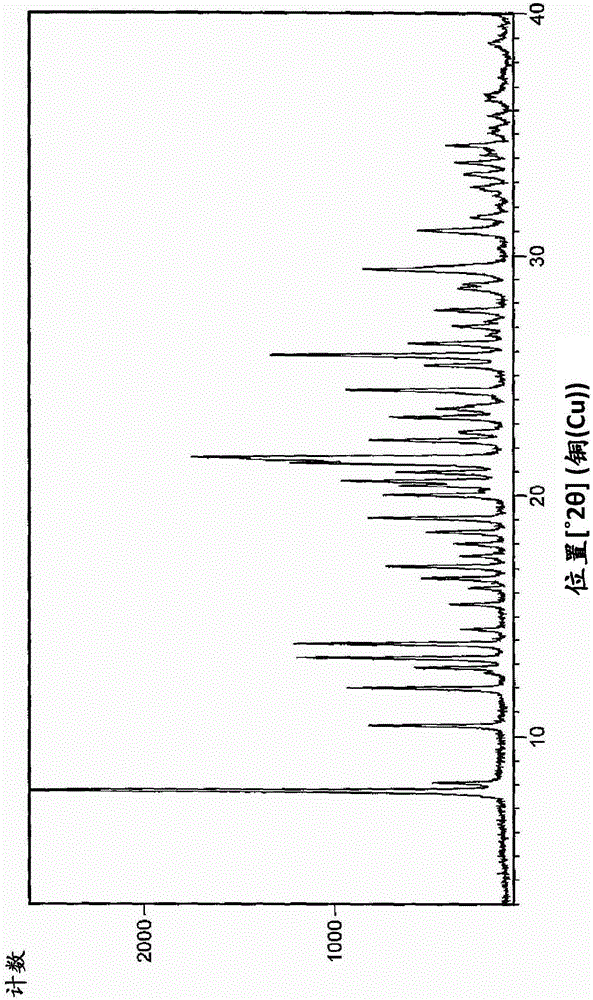
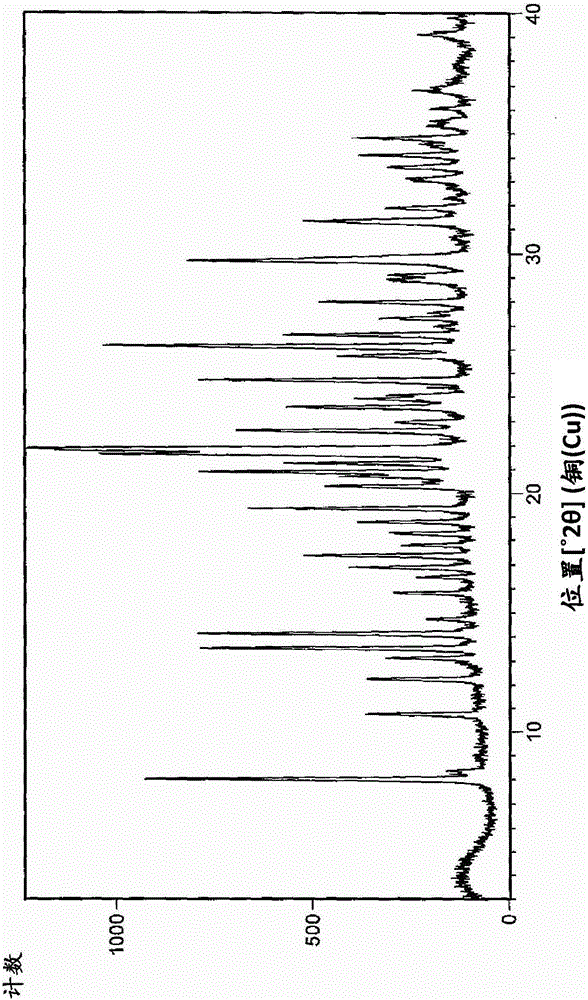
An industrially applicable process for preparing high purity aclidinium bromide
A kind of aclidinium bromide, high-purity technology, applied in the field of preparing aclidinium bromide of formula I, can solve problems such as long reaction time or chlorinated solvent, inconvenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1 (reference examples; program according to the method of patent EP2130830)
[0126] Fresh sodium ethoxide (43 mg, 1.2 equiv) and methyl bis(2-thienyl)glycolate VI (200 mg, 1.2 equiv) were added to R-(-)-3-quinuclidine under an inert argon atmosphere Alcohol III (63mg, 0.5mmol) was suspended in anhydrous toluene (5ml). The reaction mixture was heated for 2.5 hours (1 hour at 70°C to 80°C and 1.5 hours at 80°C to 95°C). At the same time, an azeotropic mixture consisting of toluene and methanol, a by-product of the reaction, was partially removed by distillation (1.5 ml). When the internal temperature reached 110°C to 115°C, an additional 2ml was removed by distillation. Add 4 ml of anhydrous toluene and continue distillation. Heating was stopped after 3 ml of the azeotrope had been removed by distillation. The reaction mixture was cooled in an ice bath, and 2 ml diethyl ether and 2 ml water were added. The resulting two layers were separated, and the aqueo...
Embodiment 2
[0127] Example 2 (Preparation method of quinuclidinyl ester V, experiments 1 to 4 of Table 1)
[0128] 1.5 g of bis(2-thienyl) methyl glycolate VI (5.9 mmol) and 0.86 g of R-(-)-3-quinuclidinol III (1.15 equivalents) were weighed into a flask, and placed in an inert argon atmosphere Add 20mlMeTHF to it. The reaction mixture was heated to 35 °C and a 1 M solution of tert-butoxide (sodium tert-butoxide or potassium tert-butoxide according to Table 1) in MeTHF was added dropwise to the reaction mixture under an inert argon atmosphere over 20 min (0.5 equivalent). After adding the base, a distillation apparatus was fitted to the flask, and the reaction mixture was gradually heated to 80° C. (the internal temperature of the reaction mixture was 70° C.) and stirred further at this temperature for the time mentioned in Table 1. During this period, the azeotrope consisting of MeTHF and methanol, a by-product of the reaction, was simultaneously removed by distillation under reduced...
Embodiment 3
[0129] Example 3 (Preparation method of quinuclidinyl ester V, experiments 5 to 7 of Table 1)
[0130] 1.5 g of bis(2-thienyl) methyl glycolate VI (5.9 mmol) and 0.86 g of R-(-)-3-quinuclidinol III (1.15 equivalents) were weighed into a flask, and placed in an inert argon atmosphere Add 20mlMeTHF to it. The reaction mixture was heated to 35 °C and a 1 M solution of sodium tert-butoxide in MeTHF (amount of base specified in Table 1) was added dropwise to the reaction mixture under an inert argon atmosphere over 20 min. After adding the base, a distillation apparatus was fitted to the flask, and the reaction mixture was gradually heated to 80° C. (the internal temperature of the reaction mixture was 70° C.) and stirred further at this temperature for the time mentioned in Table 1. During this period, the azeotrope consisting of MeTHF and methanol, a by-product of the reaction, was simultaneously removed by distillation under reduced pressure. The total amount of azeotrope re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com