Hydrolysis method of capsular polysaccharide of different serotypes of Streptococcus pneumoniae
A technology of Streptococcus pneumoniae and capsular polysaccharide, which is applied in the directions of antibacterial drugs, multivalent vaccines, bacterial antigen components, etc., can solve the difficult operation of the preparation and purification of polysaccharide activated conjugates, poor immune effect, and viscosity of polysaccharide solution. big problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11、2、3、4、18
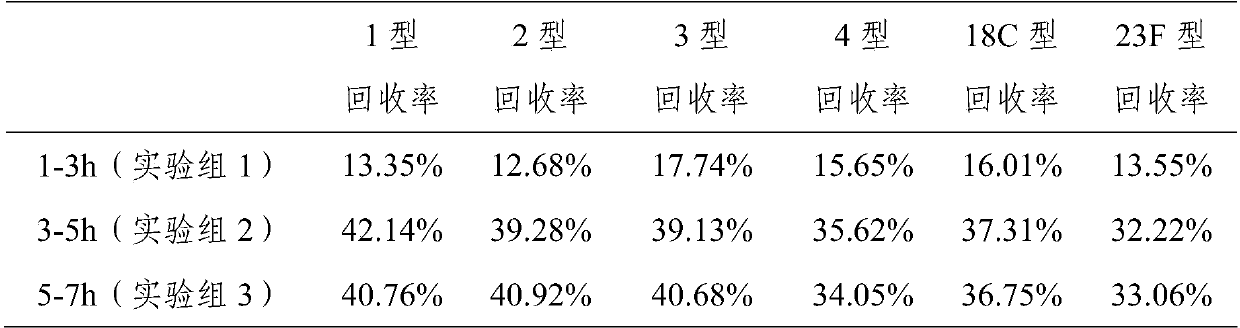
[0021] Example 1 Hydrolysis of 1, 2, 3, 4, 18C, 23F pneumococcal capsular polysaccharide
[0022] Dissolve polysaccharide 1-5mg with water for injection, make it dissolve completely, add the hydrogen peroxide that final concentration is 3% and final concentration and be 1mM zinc sulfate solution (the mass ratio that is equivalent to capsular polysaccharide, hydrogen peroxide and zinc sulfate is 1 -5:9:0.16), the reaction temperature is 50°C-80°C, and the reaction is 3-5 hours. Use a 10kD ultrafiltration membrane to carry out ultrafiltration concentration, 0.85% sodium chloride solution ultrafiltration and change the liquid, change the liquid 5 times, each time 8 times of volume dilution. Concentrate to 5 times the volume of the reaction mixture, purify with a Sepharose 4FF gel chromatography column, collect the separated peaks with a kD value of 0.2-0.6, collect and concentrate by ultrafiltration to the original loading volume, and freeze-dry to recover the capsular polysaccha...
Embodiment 2
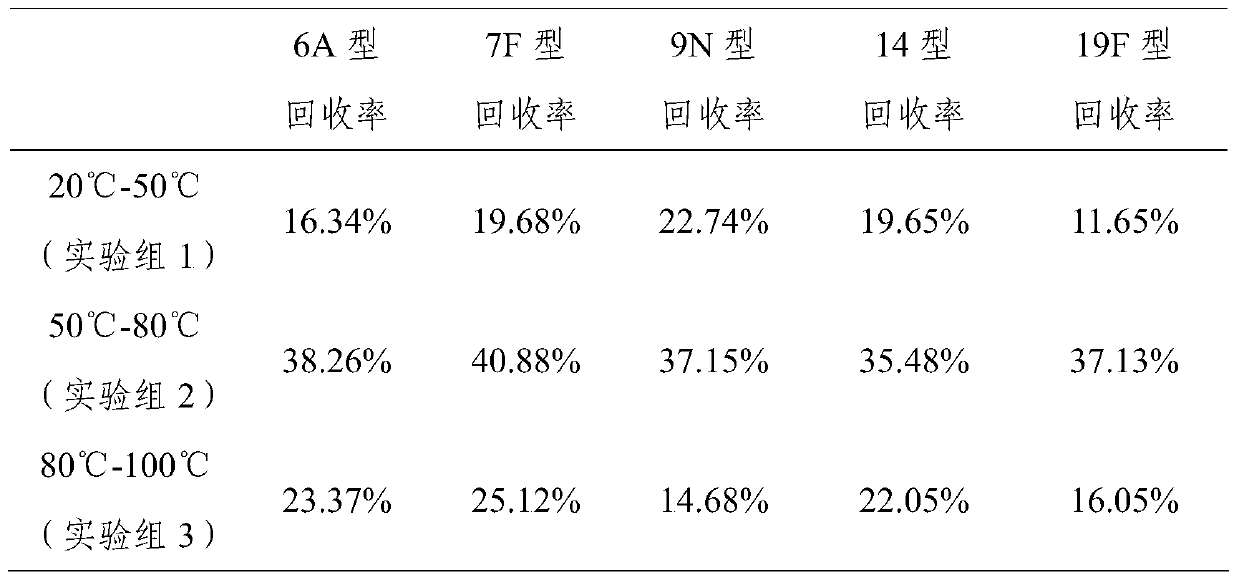
[0023] Example 2 Hydrolysis of 6A, 7F, 9N, 14, 19F pneumococcal capsular polysaccharide
[0024] Dissolve polysaccharide 1-5mg with water for injection, make it dissolve completely, add the hydrogen peroxide that final concentration is 2% and final concentration be 1mM zinc sulfate solution (the mass ratio that is equivalent to capsular polysaccharide, hydrogen peroxide and zinc sulfate is 1 -5:6:0.161), the reaction temperature is 50°C-80°C, and the reaction is 3-5 hours. Use a 10kD ultrafiltration membrane to carry out ultrafiltration concentration, 0.85% sodium chloride solution ultrafiltration and change the liquid, change the liquid 5 times, each time 8 times of volume dilution. Concentrate to 5 times the volume of the reaction mixture, purify with a Sepharose 4FF gel chromatography column, collect the separated peaks with a kD value of 0.3-0.6, collect and concentrate by ultrafiltration to the original loading volume, and freeze-dry to recover the capsular polysaccharide...
Embodiment 3
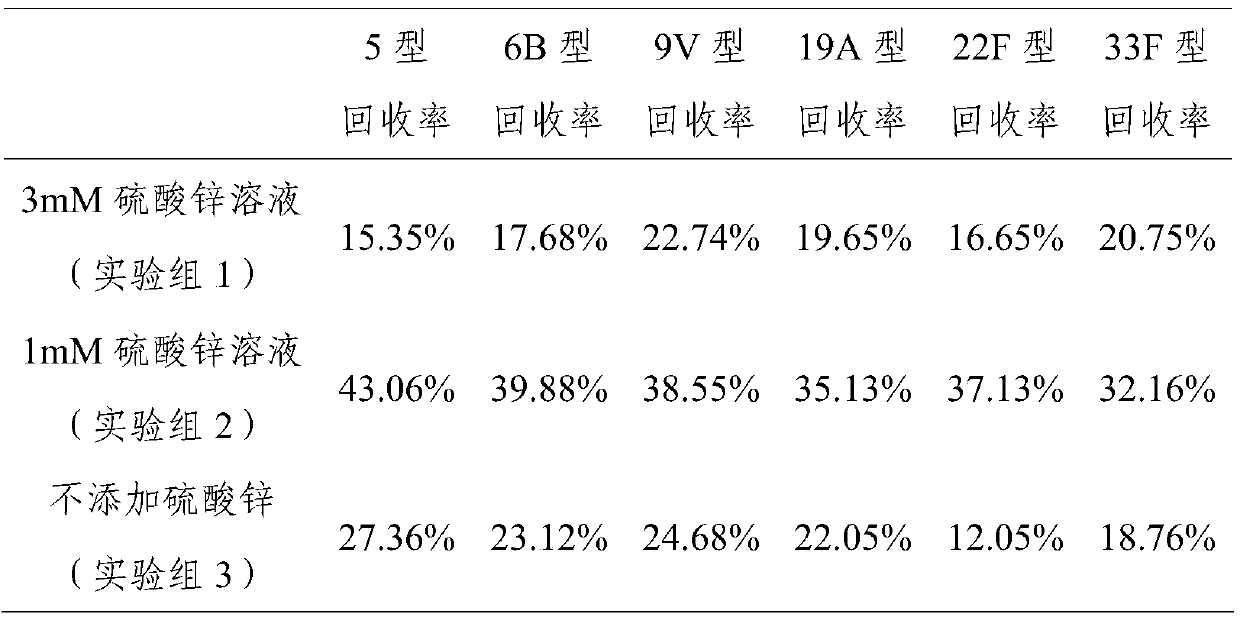
[0025] Example 3 Hydrolysis of 5, 6B, 9V, 19A, 22F, 33F type pneumococcal capsular polysaccharide
[0026] Dissolve polysaccharide 1-5mg with water for injection, make it dissolve completely, add the hydrogen peroxide that final concentration is 1.5% and final concentration be 1mM zinc sulfate solution (the mass ratio that is equivalent to capsular polysaccharide, hydrogen peroxide and zinc sulfate is 1 -5:4.5:0.161), the reaction temperature is 50°C-80°C, and the reaction is 3-5 hours. Use a 10kD ultrafiltration membrane to carry out ultrafiltration and concentrate 0.85% sodium chloride solution and change the liquid by ultrafiltration, and change the liquid 5 times, each time 8 times of volume dilution. Concentrate to 5 times the volume of the reaction mixture, purify with Sepharose4FF gel chromatography, collect the separated peaks with a kD value of 0.35-0.65, collect and concentrate by ultrafiltration to the original loading volume, and freeze-dry to recover the hydrolysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com