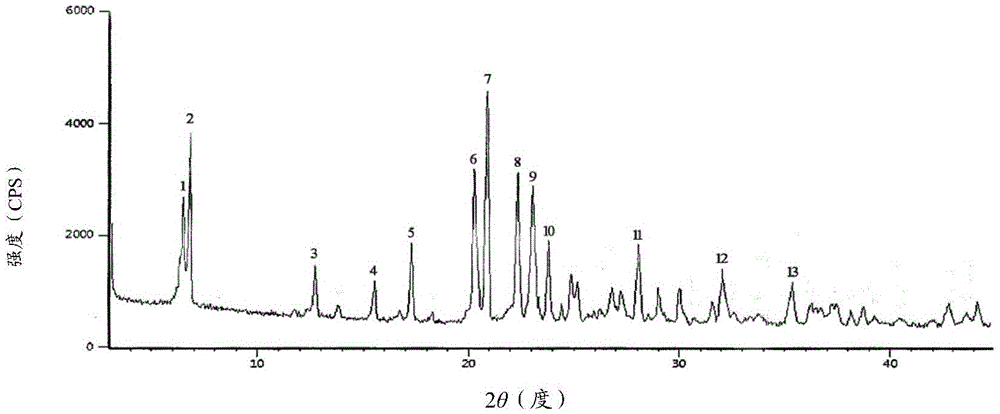
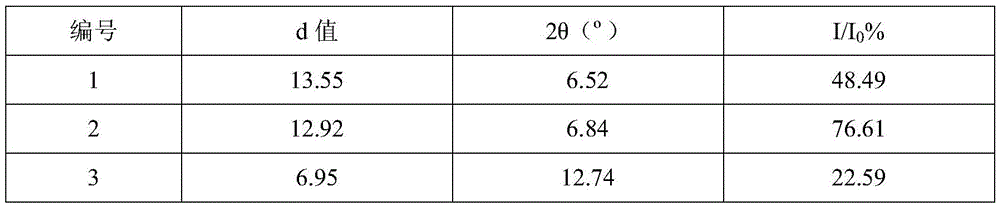
Cefuroxime sodium new crystal type compound and preparation adopting particle process crystal product molecular assembly and form optimizing technology
A technology of cefuroxime sodium and compound, applied in the field of medicine, can solve the problems of cefuroxime sodium, such as deep color, instability, content reduction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of Cefuroxime Sodium New Crystal Form Compound
[0026] (1) Dissolve 54.4g of 7-ACA in 1000ml of chloroform, under stirring, add 60.6g of triethylamine to the above solution, stir for 30min, slowly add 50.1g of SMIF-Ts, keep warm at 0-5°C, and react 2h, add 500ml of distilled water, stir and react for 10min, take the water layer, decolorize with 5g of activated carbon at room temperature for 10min, filter, cool the filtrate to 0-5°C, slowly add 2mol / L hydrochloric acid solution, adjust the pH value to 3.0, precipitate crystals, and stir for 1h , filtered, washed with ice water until neutral, and dried under vacuum at 40°C to obtain 74.9g of 7-FCA.
[0027] (2) Add 70.0g of 7-FCA and 600ml of methanol to the reaction bottle, cool to 0-5°C under stirring, slowly add 700ml of 4% NaCO3 aqueous solution, after the addition, warm up to 10°C for 30min, and extract with ethyl acetate 2 times, 1000ml each time, combined the organic layers, washed 2 times ...
Embodiment 2
[0037] Embodiment 2: Preparation of new crystal form compound of cefuroxime sodium
[0038] (1) Dissolve 53.8g of 7-ACA in 1000ml of chloroform, and add 61.5g of triethylamine to the above solution under stirring. After stirring for 30min, slowly add 52.1g of SMIF-Ts, keep warm at 0-5°C, and react 2h, add 500ml of distilled water, stir and react for 10min, take the water layer, decolorize with 5g of activated carbon at room temperature for 10min, filter, cool the filtrate to 0-5°C, slowly add 2mol / L hydrochloric acid solution to adjust the pH to 3.1, precipitate crystals, stir for 1h, filter , washed with ice water until neutral, and dried under vacuum at 40°C to obtain 75.8g of 7-FCA.
[0039] (2) Add 70.5g of 7-FCA and 600ml of methanol into the reaction bottle, cool to 0-5°C under stirring, slowly add 700ml of 4% NaCO3 aqueous solution, after the addition, warm up to 10°C for 30min, and extract with ethyl acetate 2 times, 1000ml each time, combine the organic layers, wash ...
Embodiment 3
[0043] Embodiment 3: Preparation of new crystal form compound of cefuroxime sodium
[0044] (1) Dissolve 50.5g of 7-ACA in 1000ml of chloroform, and add 51.5g of triethylamine to the above solution under stirring. After stirring for 30min, slowly add 51.1g of SMIF-Ts, keep warm at 0-5°C, and react 2h, 500ml of distilled water was added, and the reaction was stirred for 10min. Take the water layer, decolorize with 5g of activated carbon at room temperature for 10min, filter, cool the filtrate to 0-5°C, slowly add 2mol / L hydrochloric acid solution to adjust the pH value to 3.3, precipitate crystals, stir for 1h, filter, wash with ice water until neutral, 40 °C and vacuum-dried to obtain 74.9 g of 7-FCA.
[0045] (2) Add 70.1g of 7-FCA and 600ml of methanol to the reaction bottle, cool to 0-5°C under stirring, slowly add 700ml of 4% NaCO3 aqueous solution, after the addition, warm up to 10°C for 30min, and extract with ethyl acetate 2 times, 1000ml each time, combine the organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffraction angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com