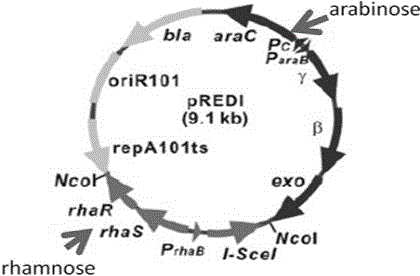
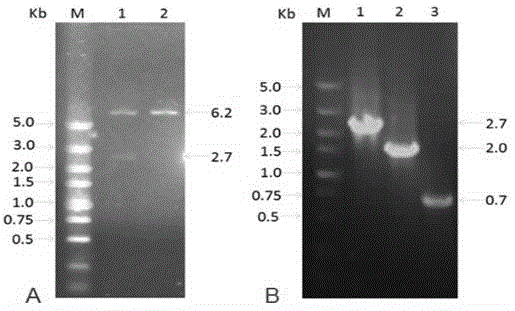
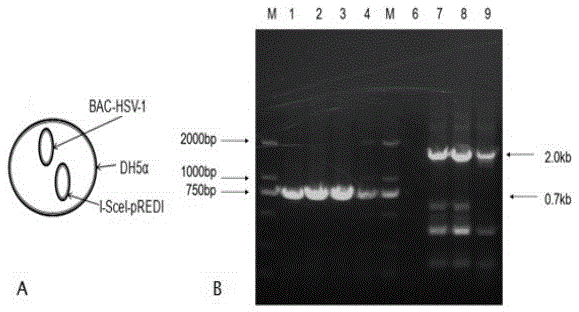
Herpes simplex virus I gene recombinant attenuated live vaccine and preparation method thereof
A technology of herpes simplex virus and live attenuated vaccine, which is applied in the field of biomedicine, can solve the problems of complex preparation method of live attenuated vaccine, difficult PCR amplification, low success probability, etc., and achieves improved homologous recombination efficiency and high immunity. Original, targeted effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0039] Example 1: Preliminary screening of HSV-1 attenuating genes
[0040] Using SiRNA interference technology, the effects of UL5, UL39, UL40, UL41, US6, US8, US12, UL18, UL39, ICP8, ICP34.51 and ICP34.52 genes of HSV-1 on virus proliferation and infectivity were studied. The attenuated genes that were knocked out were screened initially, and the results are shown in Table 1. The PCR primer sequences for the knockout genes UL5, US8, UL41 and UL18 are shown in SEQ ID NO. 1 to SEQ ID NO. 16, respectively, and the primer sequences for other knockout genes are not listed here. The enzyme used for the two PCR amplifications was PrimeSTARMaxDNAPolymerase (TAKARA, R045A), and the reaction system was as follows: PrimeSTARMaxPremix (2×) 25μL, upstream primer (10μM) 1.5μL, downstream primer (10μM) 1.5μL, template 2 0.25μL, add deionized water to 50μL. The reaction conditions are: 98°C for 10s, 56°C for 5s, 72°C for 5s / Kb, a total of 30 cycles, and then 72°C for 5 minutes and 4°C heat pr...
Example Embodiment
[0043] Example 2 Construction of HSV-1UL18 gene knockout virus strain
[0044] The HSV-1UL18 gene knockout virus strain was successfully constructed using the gene knockout system of the present invention. The preparation method includes:
[0045] Construction of S1, BAC-HSV-1 host strain
[0046] S1.1 Expanded culture of HSV-1 virus: culture vero cells, wash the cells with PBS, add HSV-1 virus (purchased from the American Collection of Types of Microorganisms) into the cell culture flask to ensure that the virus solution completely covers the cell surface , Put the cell culture flask with virus liquid at 37℃, 5% CO 2 Incubate in the incubator for 1.5 hours, shaking every 15 minutes during the incubation; after incubation, add DMEM maintenance solution to the cell culture flask, and put it in the cell culture incubator to continue culturing to observe the cytopathic condition. When the cytopathic rate reaches 70-80%, the virus can be collected; the amplified virus is placed at -80℃...
Example Embodiment
[0057] Example 3 Construction of HSV-1US8 gene knockout virus strain
[0058] Similar to Example 2, the HSV-1US8 gene knockout virus strain was constructed using the BAC gene knockout system.
[0059] Use primers (shown in SEQIDNO.5 to SEQIDNO.8) to PCR amplify the homologous recombination frame of the knockout gene US8. The homologous recombination frame includes the 50bp homologous sequence upstream and downstream of the target gene to be knocked out, kanamycine gene and SacB Gene, so that the kanamycine gene and SacB gene are located between the homologous flanking sequences. The electropherogram of the PCR amplified △US8 gene knockout homologous recombination frame is as follows Figure 7 Shown, where, Figure 7 -A refers to the verification electropherogram of the kanamycine+SacB recombinant fragment, Figure 7 -B refers to the verification electropherogram of the homologous fragment of the downstream homologous sequence of the kanamycine+SacB+ knockout gene US8, Figure 7 -C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com