Desloratadine citrate disodium oral rapidly disintegrating film and preparation method thereof
A technology of oral instant film and loratadine, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of children who are difficult to take, difficult to take, slow onset, etc. problems, achieve considerable economic and social benefits, rapid onset of effect, and small amount of auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
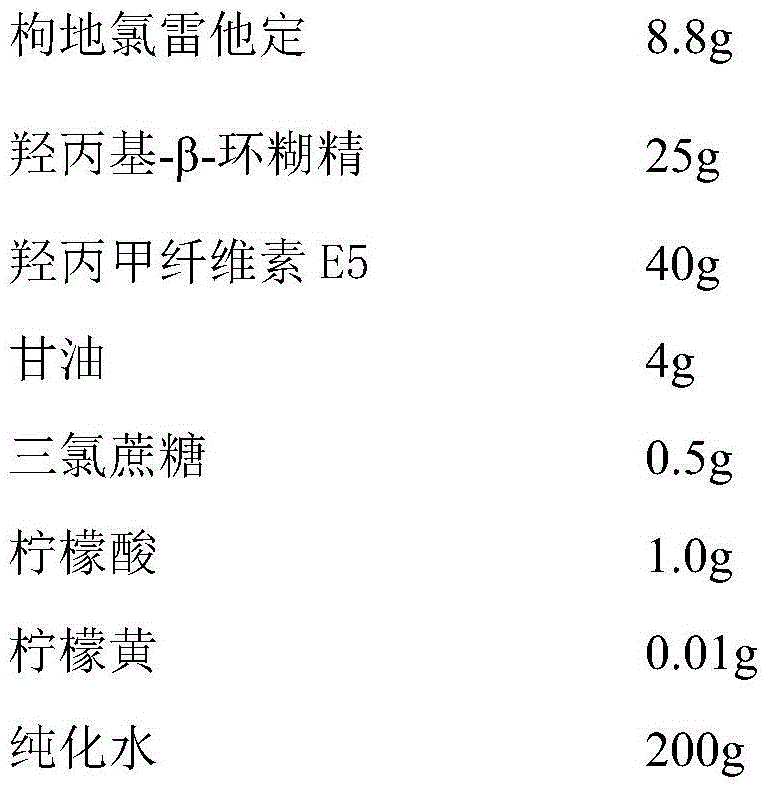
[0031] Each tablet contains 8.8 mg of desloratadine citrate, and the composition of 1000 tablets of desloratadine citrate oral instant film is:
[0032]
[0033] The preparation method is as follows:
[0034] (1) Dissolve the prescribed amount of sulfobutyl ether-β-cyclodextrin in purified water of 80% of the total amount of prescribed water, heat to 45°C under stirring, slowly add the prescribed amount of desloratadine citrate , continue stirring for 24 hours;
[0035] (2) Weigh the prescribed amount of sucralose and citric acid, add them to the solution in step (1), and stir until dissolved.
[0036] (3) Weigh the hypromellose E5 and glycerin of prescription quantity, join in the solution of step (2), wait to dissolve completely, let stand overnight, obtain the gel for scraping; Scrape the gel for scraping Apply to a glass plate, dry, then peel off the film from the glass plate and cut.
[0037] (4) Packing the cut film to obtain desloratadine citrate oral instant film...
Embodiment 2
[0040] Each tablet contains 8.8 mg of desloratadine citrate, and the composition of 1000 tablets of desloratadine citrate oral instant film is:
[0041]
[0042] The preparation method is as follows:
[0043] (1) The hydroxypropyl-beta-cyclodextrin of recipe quantity is dissolved in the purified water of 80% of prescription water total amount, is heated to 45 ℃ under stirring state, slowly adds the desloratadine citrate of recipe quantity, Continue stirring for 24 hours;
[0044](2) Weigh the prescribed amount of sucralose and citric acid, add them to the solution in step (1), and stir until dissolved.
[0045] (3) Weigh the hypromellose E5 and glycerin of prescription quantity, join in the solution of step (2), wait to dissolve completely, let stand overnight, obtain the gel for scraping; Scrape the gel for scraping Apply to a glass plate, dry, then peel off the film from the glass plate and cut.
[0046] (4) Packing the cut film to obtain desloratadine citrate oral ins...
Embodiment 3
[0049] Each tablet contains 8.8 mg of desloratadine citrate, and the composition of 1000 tablets of desloratadine citrate oral instant film is:
[0050]
[0051]
[0052] The preparation method is as follows:
[0053] (1) Dissolve the prescribed amount of sulfobutyl ether-β-cyclodextrin in purified water of 80% of the total amount of prescribed water, heat to 45°C under stirring, slowly add the prescribed amount of desloratadine citrate , continue stirring for 24 hours;
[0054] (2) Weigh the prescribed amount of sucralose and citric acid, add them to the solution in step (1), and stir until dissolved.
[0055] (3) Weigh the hypromellose E5 and glycerin of prescription quantity, join in the solution of step (2), wait to dissolve completely, let stand overnight, obtain the gel for scraping; Scrape the gel for scraping Apply to a glass plate, dry, then peel off the film from the glass plate and cut.
[0056] (4) Packing the cut film to obtain desloratadine citrate oral ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com