Sodium valproate oral preparation slow-release preparation and preparation method thereof
A technology of sodium valproate and oral preparations, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, anhydride/acid/halide active ingredients, etc., which can solve the problems of poor taste and difficulty in taking it by patients, and achieve the preparation method Simple, convenient medication, and the effect of reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
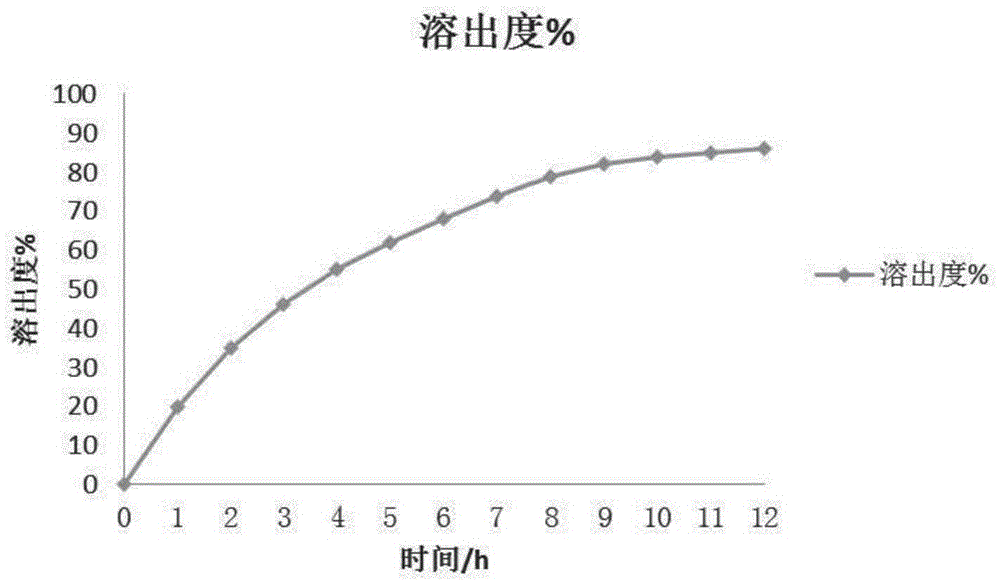
Image
Examples
Embodiment 1
[0023] formula:
[0024]
[0025] Preparation Process:
[0026] 1) Preparation of drug-containing resin: add ion-exchange resin to 700 mg / ml sodium valproate aqueous solution at room temperature, stir at constant temperature, take samples regularly, and measure the concentration of drug in the solution. After reaching equilibrium, wash off the unbound drug on the surface of the resin with deionized water, and dry at 40-60°C to obtain the drug-loaded resin;
[0027] 2) Preparation of drug-containing microcapsules: use fluidized bed bottom spray coating, put the drug resin into the fluidization chamber, adjust the air volume to make the particles in an ideal fluidization state in the fluidization chamber, control the temperature at 20°C, and keep The flow pump pumps the coating solution containing plasticizer and microcapsule material at a constant speed, so that the coating solution has a good atomization effect, and there is no intermittent drying time for continuous coati...
Embodiment 2
[0030] formula:
[0031]
[0032] Preparation Process:
[0033] 1) Preparation of drug-containing resin: add ion-exchange resin to 800 mg / ml sodium valproate aqueous solution at room temperature, stir at constant temperature, take samples regularly, and measure the concentration of drug in the solution. After reaching equilibrium, wash off the unbound drug on the surface of the resin with deionized water, and dry at 40-60°C to obtain the drug-loaded resin;
[0034] 2) Preparation of drug-containing microcapsules: use fluidized bed bottom spray coating, put the drug resin into the fluidization chamber, adjust the air volume to make the particles in an ideal fluidization state in the fluidization chamber, control the temperature at 35°C, and keep The flow pump pumps the coating solution containing plasticizer and microcapsule material at a constant speed, so that the coating solution has a good atomization effect, and there is no intermittent drying time for continuous coating...
Embodiment 3
[0037] formula:
[0038]
[0039] Preparation Process:
[0040] 1) Preparation of drug-containing resin: add ion-exchange resin to 900 mg / ml sodium valproate aqueous solution at room temperature, stir at constant temperature, take samples regularly, and measure the concentration of drug in the solution. After reaching equilibrium, wash off the unbound drug on the surface of the resin with deionized water, and dry at 40-60°C to obtain the drug-loaded resin;
[0041] 2) Preparation of drug-containing microcapsules: spray coating at the bottom of the fluidized bed, put the drug resin into the fluidization chamber, adjust the air volume to make the particles in an ideal fluidization state in the fluidization chamber, control the temperature at 40°C, and keep The flow pump pumps the coating solution containing plasticizer and microcapsule material at a constant speed, so that the coating solution has a good atomization effect, and there is no intermittent drying time for contin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com