A ligand for metal-organic framework and its synthesis method
A metal-organic framework and a synthesis method technology, applied in the field of C3 symmetrical aromatic tricarboxylic acid ligands and their synthesis, can solve the problems of no pore structure, limited application, etc., and achieve easy purification, high yield, coordination Mode flexible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
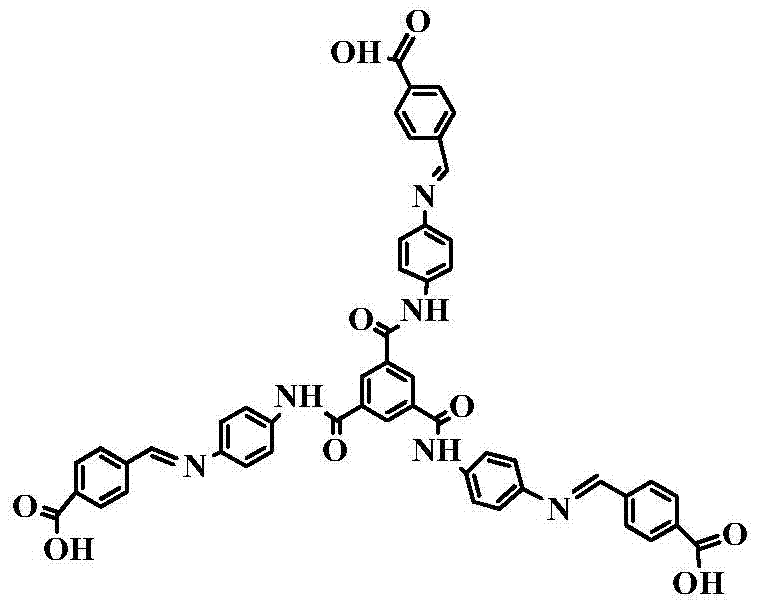
[0032] A ligand for metal-organic frameworks, named C 3 The symmetrical aromatic tricarboxylic acid ligand is a semi-rigid Schiff base structure composed of multiple benzene rings; it contains O=C-NH-functional groups; its appearance is yellow solid powder; its structure is as follows:
[0033]
[0034] The concrete steps of its synthetic method are as follows:
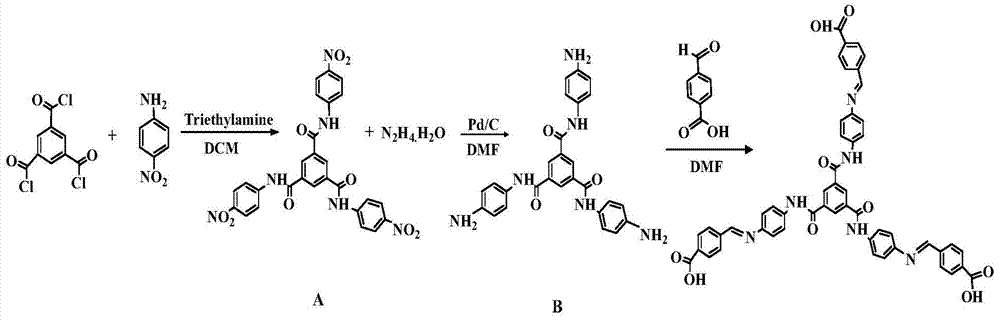
[0035] Step 1, the preparation of intermediate product A
[0036] Add 30mL of dichloromethane solution containing 1.57g (11.4mmol) p-nitroaniline to a 100mL container, add 1.60mL triethylamine as an acid-binding agent, slowly add 20mL solution of 1.00g (3.79 mmol) of 1,3,5-benzenetricarbonyl chloride in dichloromethane, reacted for 4 hours, filtered to obtain a light yellow-green solid, washed several times with ethanol solution, and dried in vacuo to finally obtain 1.90 g of the product with a yield of 88.0%.
[0037] Step 2, the preparation of intermediate product B
[0038] Add 20 mL of DMF solution in which ...
Embodiment 2
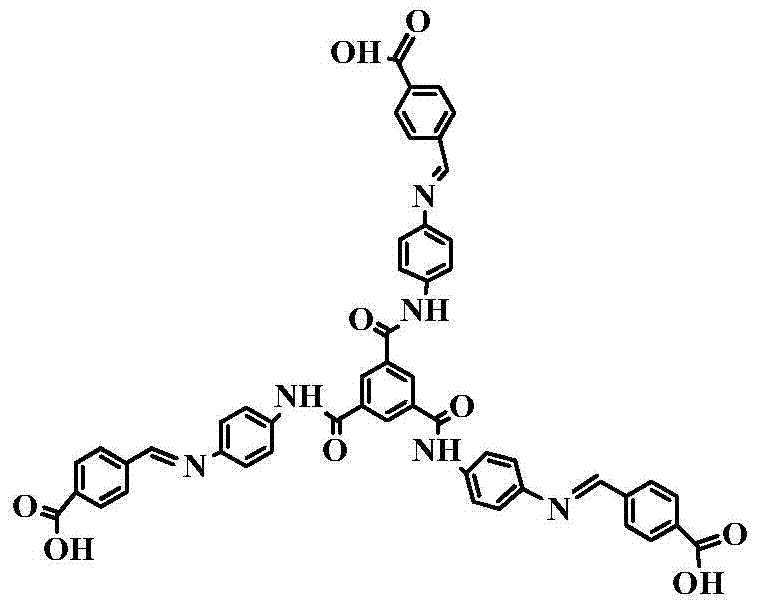
[0042] A ligand for metal-organic frameworks, named C 3 The symmetrical aromatic tricarboxylic acid ligand is a semi-rigid Schiff base structure composed of multiple benzene rings; it contains O=C-NH-functional groups; its appearance is yellow solid powder; its structure is as follows:
[0043]
[0044] The concrete steps of its synthetic method are as follows:
[0045] Step 1, the preparation of intermediate product A
[0046] Add 50mL of dichloromethane solution containing 2.48g (18.0mmol) of p-nitroaniline into a 100mL container, add 1.1mL of pyridine as an acid-binding agent, slowly add 10mL of 0.50g (1.90mmol) of p-nitroaniline dropwise under stirring in an ice bath The dichloromethane solution of 1,3,5-benzenetricarboxylic acid chloride was reacted for 3 hours to obtain a light yellow-green solid, which was washed several times with ethanol solution and then vacuum-dried to obtain 0.90 g of the product with a yield of 84.9%.
[0047] Step 2, the preparation of inter...
Embodiment 3
[0052] A ligand for metal-organic frameworks, named C 3 The symmetrical aromatic tricarboxylic acid ligand is a semi-rigid Schiff base structure composed of multiple benzene rings; it contains O=C-NH-functional groups; its appearance is yellow solid powder; its structure is as follows:
[0053]
[0054] The concrete steps of its synthetic method are as follows:
[0055] Step 1, the preparation of intermediate product A
[0056] Add 20 mL of dichloromethane solution containing 1.24 g (9.0 mmol) p-nitroaniline into a 50 mL container, add 2.3 mL of pyridine as an acid-binding agent, slowly add 10 mL of 0.50 g (1.90 mmol) The dichloromethane solution of 1,3,5-benzenetricarboxylic acid chloride was reacted for 2 hours to obtain a light yellow-green solid, which was washed several times with ethanol solution and then vacuum-dried to obtain 0.96 g of the product with a yield of 88.6%.
[0057] Step 2, the preparation of intermediate product B
[0058] Add 20 mL of DMF solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com