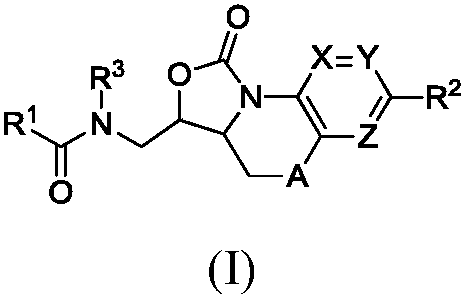
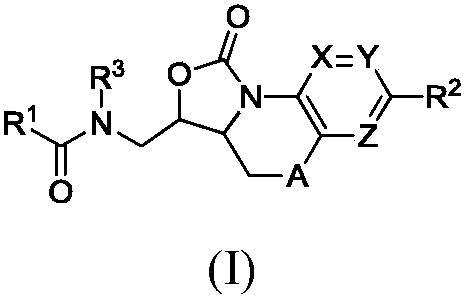
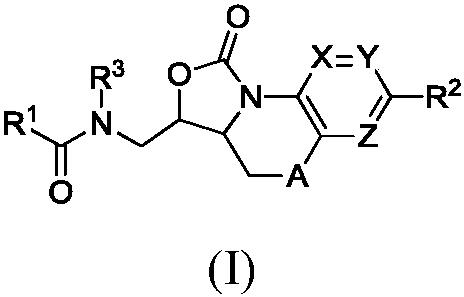
Benzoxazolooxazinones as factor xa inhibitors
A kind of compound, the technology of phenyl, be applied in the field of new benzoxazolo oxazinone compound, can solve the problem such as no teaching, do not have coagulation factor Xa inhibition effect etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] 5-chloro-N-(((3S,3aS)-1-oxo-7-(3-oxomorpholino)-1,3,3a,4-tetrahydrobenzo[b]oxazolo [3,4-d][1,4]oxazin-3-yl)methyl)thiophene-2-carboxamide
[0222]
[0223] Reaction flow:
[0224]
[0225] Step A: Cool a solution of (Z)-but-2-ene-1,4-diol (39.7g, 450mmol) in dry THF (300mL) to 0°C, add sodium hydride (60% dissolved in mineral oil) in batches , 9.0 g, 225 mmol), then a dry THF solution (450 mL) of 4-bromo-2-fluoro-1-nitrobenzene (33.0 g, 150 mmol) was added dropwise at 0°C. The reaction solution was stirred at room temperature for two hours, poured into 600 mL of water, the mixture was extracted with EtOAc, the combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated to give (Z)-4-(5-bromo-2-nitrobenzene oxy)but-2-en-1-ol (45 g), yellow solid. The crude product was used in the next step without further purification.
[0226] Step B: Add zinc powder (49.0g, 750mmol) and ammonium chloride (40.0g, 750mmol) into methanol (450mL)...
Embodiment 2
[0238] 5-chloro-N-(((3S,3aS)-1-oxo-7-(2-oxopiperidin-1-yl)-1,3,3a,4-tetrahydrobenzo[b]oxa azole And[3,4-d][1,4]oxazin-3-yl)methyl)thiophene-2-carboxamide
[0239]
[0240] Step A: Preparation of (3R,3aS)-3-((tert-butyldimethylsilyloxy)methyl)-7-(2-oxopiperidin-1-yl)-3α according to Example 1, 4-Dihydrobenzo[b]oxazolo[3,4-d][1,4]oxazin-1(3H)-one, 7% yield, wherein the morpholin-3 in step G -one is replaced by piperidin-2-one. LCMS (ESI) m / z: 433.2 (M+1).
[0241] Step B: The title compound of Example 2 was prepared sequentially according to Example 1 Steps G, I, J, K and L as a white solid. 1 HNMR (400MHz, DMSO-d 6 )δ9.01(t, J=5.6Hz, 1H), 7.83(d, J=8.8Hz, 1H), 7.72(d, J=4.0Hz, 1H), 7.22(d, J=4.0Hz, 1H) , 6.87-6.93(m, 2H), 4.50-4.64(m, 2H), 3.99-4.13(m, 2H), 3.73(t, J=5.6Hz, 2H), 3.52-3.60(m, 2H), 2.37 (t, J=6.0Hz, 2H), 1.77-1.90 (m, 4H); LCMS (ESI) m / z: 462.1 (M+1).
Embodiment 3
[0243] 5-chloro-N-(((3S,3aS)-1-oxo-7-(5-oxo-1,4-oxazepan-4-yl)-1,3,3a, 4- Tetrahydrobenzo[b]oxazol[3,4-d][1,4]oxazin-3-yl)methyl)thiophene-2-carboxamide
[0244]
[0245] Step A: Preparation of (3R,3aS)-3-(((tert-butyldimethylsilyl)oxy)methyl)-7-(5-oxo-1,4-oxa according to Example 1 Azepan-4-yl)-3a,4-dihydrobenzo[b]oxazolo[3,4-d][1,4]oxazin-1(3H)-one, wherein the step The morpholin-3-one in G was replaced by 1,4-oxazepan-5-one, the yield was 35%. LCMS (ESI) m / z: 449.2 (M+1).
[0246] Step B: The title compound of Example 3 was prepared sequentially according to Example 1 Steps G, I, J, K and L as a white solid. 1 H NMR (400MHz, DMSO-d 6 )δ9.01(s, 1H), 7.82(d, J=8.4Hz, 1H), 7.72(d, J=4.0Hz, 1H), 7.22(d, J=4.0Hz, 1H), 6.87-6.83( m, 2H), 4.60-4.54(m, 2H), 4.10-4.00(m, 2H), 3.80-3.65(m, 8H), 2.78(t, J=4.8Hz, 2H); LCMS (ESI) m / z: 478.1(M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com