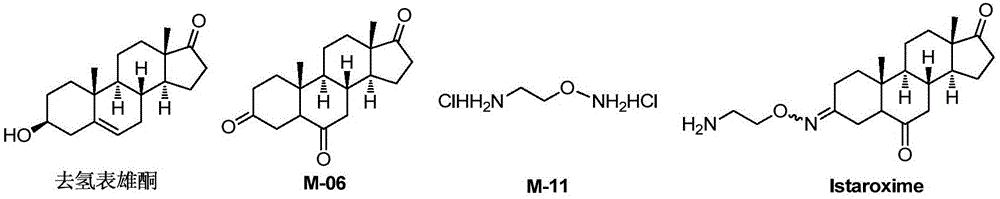
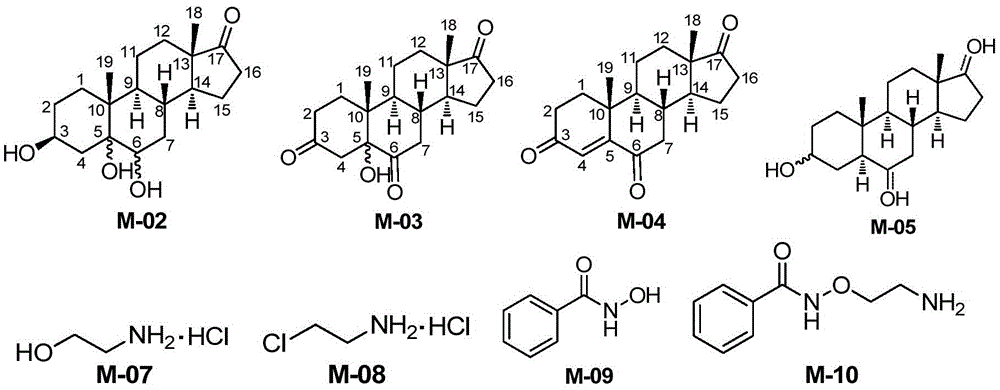
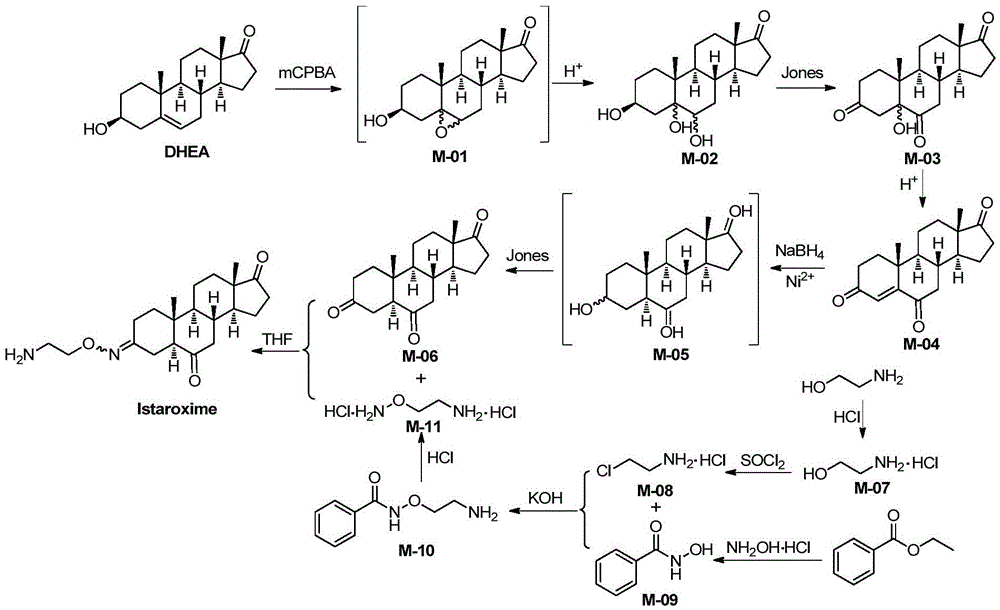
Novel synthesis method of Istaroxime
A synthesis method and the resulting technology are applied in the fields of steroids and organic chemistry, which can solve the problems of large amount of tetrahydrofuran borane complex, high temperature, and increased synthesis input costs, and achieve reduced synthesis costs, simple operation, and effective Conducive to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) Synthesis of intermediate M-02
[0075]
[0076] The reagents and reaction conditions are: i.m-CPBA, CH 2 Cl 2 ,rt; ii.H 2 SO 4 , rt.
[0077] Take dehydroepiandrosterone (DHEA, 5mmol) in the reaction bottle, add CH 2 Cl 2 After stirring and dissolving, m-chloroperoxybenzoic acid (10 mmol) was added, and the mixture was stirred at room temperature for reaction. After about 22 hours, the reaction was complete after TLC detection, and sodium sulfite ice solution was added to the reaction liquid to quench the reaction. After stirring for 5 min, 4 mL of sulfuric acid aqueous solution with a mass fraction of 20% was added, and stirred at room temperature for 12 h. The reaction solution was poured into water, filtered with suction, and the lower precipitate was removed, washed with dichloromethane, and dried to obtain 1.56 g of a white solid, M-02, with a yield of 97%.
[0078] The spectral data of M-02 is:
[0079] ESI-MS:m / z:323.1[M+H] + ;
[0080] 1 H-NMR...
Embodiment 2
[0147] The method of this example is the same as that of Example 1, the difference is:
[0148] (i), step (1) uses m-chloroperoxybenzoic acid (m-CPBA) 1.5 times the amount of dehydroepiandrosterone, reacted for 35 hours, and the yield was 79%;
[0149] (ii), the reaction temperature of step (2) is 0°C, and the yield is 93%;
[0150] (iii), step (3) uses 5 mL of 20% sulfuric acid to react for 15 hours, and the yield is 77%;
[0151] (iv), step (4) adopts nickel chloride hexahydrate 1 times the amount of M-04 substance and sodium borohydride 3 times the amount of M-04 substance to react for 3 hours, and the yield is 68%;
[0152] (v), step (5) drip concentrated hydrochloric acid with the rate of 1 drop / 2s, productive rate 99%;
[0153] (vi), step (6) uses 1.2 times the SOCl of M-07 quality 2 Reaction at 65°C for 4 hours, yield 91%;
[0154] (vii), step (7) uses the hydroxylamine hydrochloride of the amount of 1.5 times of substance and the KOH of the amount of 2 times of sub...
Embodiment 3
[0161] The method of this example is the same as that of Example 1, the difference is:
[0162] (i), step (1) uses m-chloroperoxybenzoic acid (m-CPBA) which is 4 times the amount of dehydroepiandrosterone, reacted for 15 hours, and the yield was 86%;
[0163] (ii), the reaction temperature of step (2) is 20°C, and the yield is 78%;
[0164] (iii), step (3) uses 60% sulfuric acid 2mL to react for 4 hours, and the yield is 84%;
[0165] (iv), step (4) adopts nickel chloride hexahydrate 2 times the amount of M-04 substance and sodium borohydride 7 times the amount of M-04 substance to react for 1 hour, and the yield is 81%;
[0166] (v), step (5) drip concentrated hydrochloric acid with the rate of 3D / s, productive rate 96%;
[0167] (vi), step (6) uses 2 times the SOCl of M-07 quality 2 Reaction at 85°C for 3 hours, yield 95%;
[0168] (vii), step (7) uses 2.5 times the hydroxylamine hydrochloride of the amount of potassium hydroxide substance to react, and the productive ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com