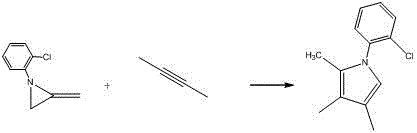Preparation method of pyrrole derivatives
A technology of pyrrole derivatives and pyrrole, applied in the direction of organic chemistry, etc., can solve the problems of difficult industrialized production, long reaction time, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
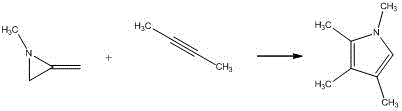
Embodiment 1
[0021]
[0022] Add 5mol% (relative to the alkyne) silver azotetrazole catalyst C in sequence in a 20ml flask 3 h 8 AgN 7 , then add 20mmol methylene aziridine and 10mmol alkyne, add 10ml toluene, and finally react at room temperature 25°C for 2h. After the reaction was completed, the reaction was filtered, the solvent was concentrated and evaporated by a rotary evaporator, the solid was subjected to silica gel column chromatography, and the column was washed with an eluent of petroleum ether:ethyl acetate=20:1 to obtain the target pyrrole compound with a yield of 90%. Purity≥99%.
Embodiment 2
[0024]
[0025] Add 4 mol% (relative to the alkyne) silver azotetrazole catalyst C in a 20ml flask 3 h 8 AgN 7 , Then add 20mmol methylene aziridine and 10mmol alkyne, add 10ml toluene, and finally react at room temperature 25°C for 5h. After the reaction was completed, it was filtered, and the solvent was concentrated and evaporated by a rotary evaporator. The solid was subjected to silica gel column chromatography, and the column was washed with an eluent of petroleum ether:ethyl acetate=20:1 to obtain the target pyrrole compound with a yield of 92%. Purity≥99%.
Embodiment 3
[0027]
[0028] Add 3 mol% (relative to the alkyne) silver azotetrazole catalyst C in a 20ml flask 3 h 8 AgN 7 , Then add 20mmol methylene aziridine and 15mmol alkyne, add 15ml toluene, and finally react at room temperature 25°C for 6h. After the reaction was completed, it was filtered, and the solvent was concentrated and evaporated by a rotary evaporator. The solid was subjected to silica gel column chromatography, and the column was washed with an eluent of petroleum ether:ethyl acetate=20:1 to obtain the target pyrrole compound with a yield of 92%. Purity≥99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com