Short peptides PCP7 specifically binding to prostatic cancer cells and application of short peptides PCP7
A prostate cancer and prostate technology, applied in the field of protein peptides, can solve the problem of few reports on prostate cancer targeting ligands, and achieve the effects of small molecular weight, good tissue penetration, and high diagnostic sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Screening of Prostate Cancer-Specific Binding Polypeptides
[0020] In this embodiment, a phage display random 12-peptide library is used to subtractively screen the polypeptides that specifically bind to prostate cancer cells, and the specific steps are as follows:
[0021] After the human prostate cancer cells LNCaP and PC3 were digested with trypsin, the cell density was adjusted, seeded in a culture dish pre-coated with polylysine, and the screening experiment was carried out when the cells grew to 80%-90% confluence.
[0022] Take the above LNCaP and PC3 cells, culture them with serum-free DMEM, add bovine serum albumin BSA to block, then add 20 μl phage peptide library, incubate for 1.5 h, pour to remove unbound phage, and shake upside down to remove residual solution. Rinse 4 times with washing solution 0.2% (v / v) TBST buffer, add non-specific buffer 0.2M glycine-hydrochloric acid (pH2.2) 2ml, suck out the eluate, add 250μl 5MTris-HCl (pH9.0) to neutral...
Embodiment 2
[0027] The amplification of embodiment 2 phage
[0028] In Example 1, the phage obtained in each round of screening was diluted 100 times with LB medium, and 20 μl of the diluted phage was mixed with 200 μl of E. After the top layer of agar, quickly pour it on the LB solid plate containing IPTG / Xgal, treat it for 24 hours, count the number of plaques on the plate where the phage can grow, and then multiply this number by the dilution factor to get the plaque forming unit (pfu) per 10 μl phage Titer.
[0029] Enrichment of phage polypeptides specifically binding to prostate cancer cells: As shown in Table 1, the positive phage clones binding to LNCaP and PC3 increased the phage recovery rate by 1000 times after four rounds of selection.
[0030] Table 1 The recovery rate of positive phage after four rounds of screening
[0031] Screening times
Embodiment 3
[0032] Embodiment 3ELISA identification phage polypeptide
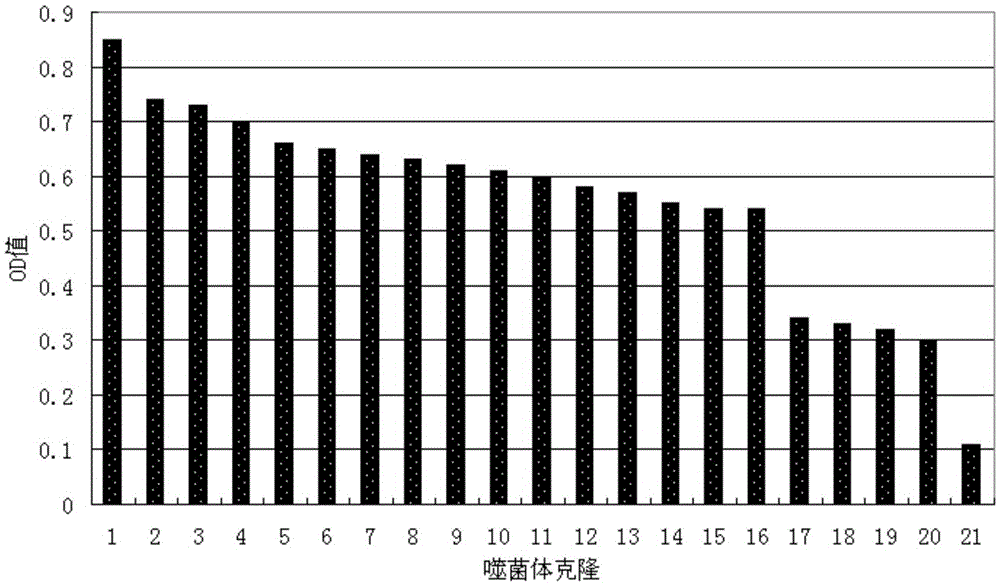
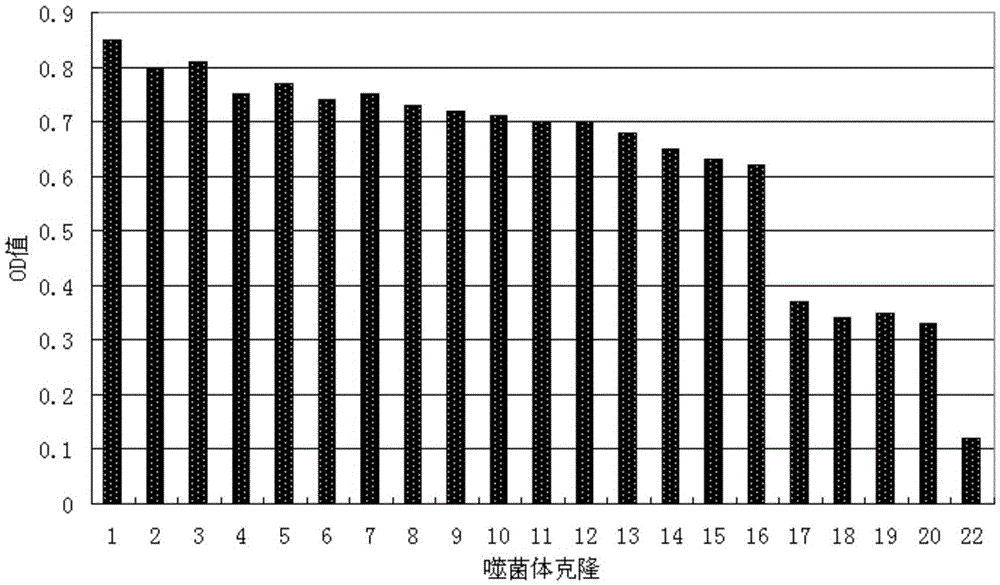
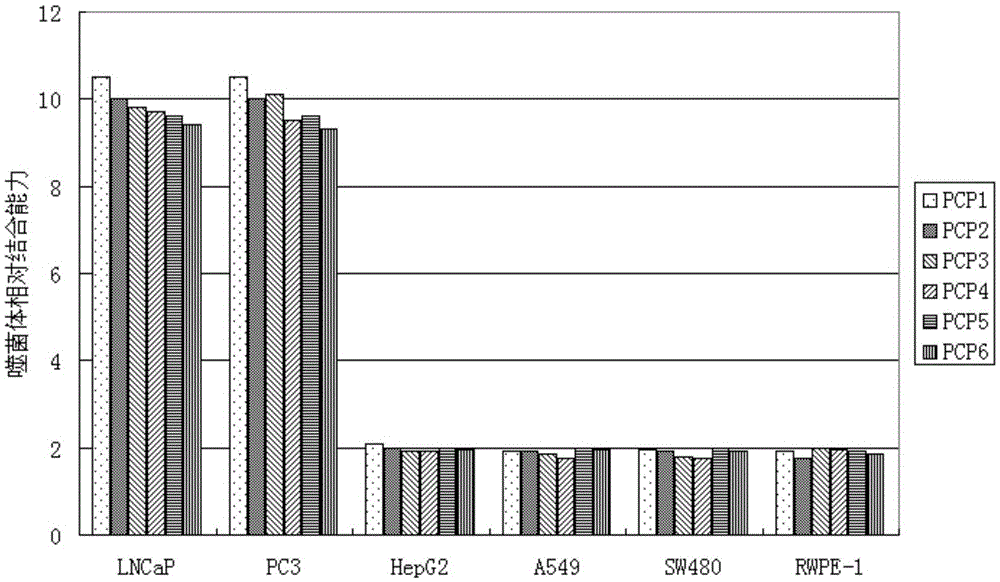
[0033] Identification of positive clones of phage polypeptides specifically binding to prostate cancer: In Example 1, after four consecutive rounds of subtractive screening of the phage peptide library, 20 phage clones were randomly selected, and the conventional methods in this field were used to preliminarily identify the phage clones against LNCaP. and PC3 affinity.
[0034] Divide LNCaP and PC3 by 1×10 4 The density per well was seeded in a 96-well plate and placed in CO 2 After culturing in the incubator for 20 hours, the cells were treated with serum-free for 1 hour, washed, fixed with paraformaldehyde, washed once with PBS, treated with TritonX-100, blocked with PBS-BSA, added phage monoclonal, incubated for 2 hours; added HRP-antiM13 Antibody, incubate at 37°C for 1.5h; develop color with TMB, add an equal volume of 2NHCl or 1NH 2 SO 4 To terminate the reaction, the reaction solution in the microplate well...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com