Preparation method of methimazole
A technology of methimazole and methimazole, which is applied in the field of drug synthesis, can solve the problems that methimazole needs to be improved, and achieve the effects of eliminating extraction and washing steps, easy post-processing, and improving reaction yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] Concrete preparation method of the present invention is as follows:
[0021] N-methylimidazole is dissolved in tetrahydrofuran, and n-butyllithium-n-hexane solution is added dropwise at -15°C to 0°C, and then incubated for 0.5 to 2 hours. Then add elemental sulfur in batches at this temperature, heat up and reflux for 6-10 hours after the addition is complete, no raw material spots are detected by thin-layer chromatography, then quench the reaction in an ice-water bath, add dilute hydrochloric acid to adjust the pH to 5.0-7.0, and then The reaction system was concentrated to dryness. The concentrated solid was added to ethyl acetate and activated carbon, heated and dissolved, stirred at reflux for 1 hour, filtered, and the filtrate was concentrated under reduced pressure to obtain methimazole.
[0022] The synthetic route of the present invention is as follows:
[0023]
[0024] We surprisingly found that N-methylimidazole, n-butyllithium and sulfur powder particip...
Embodiment 1
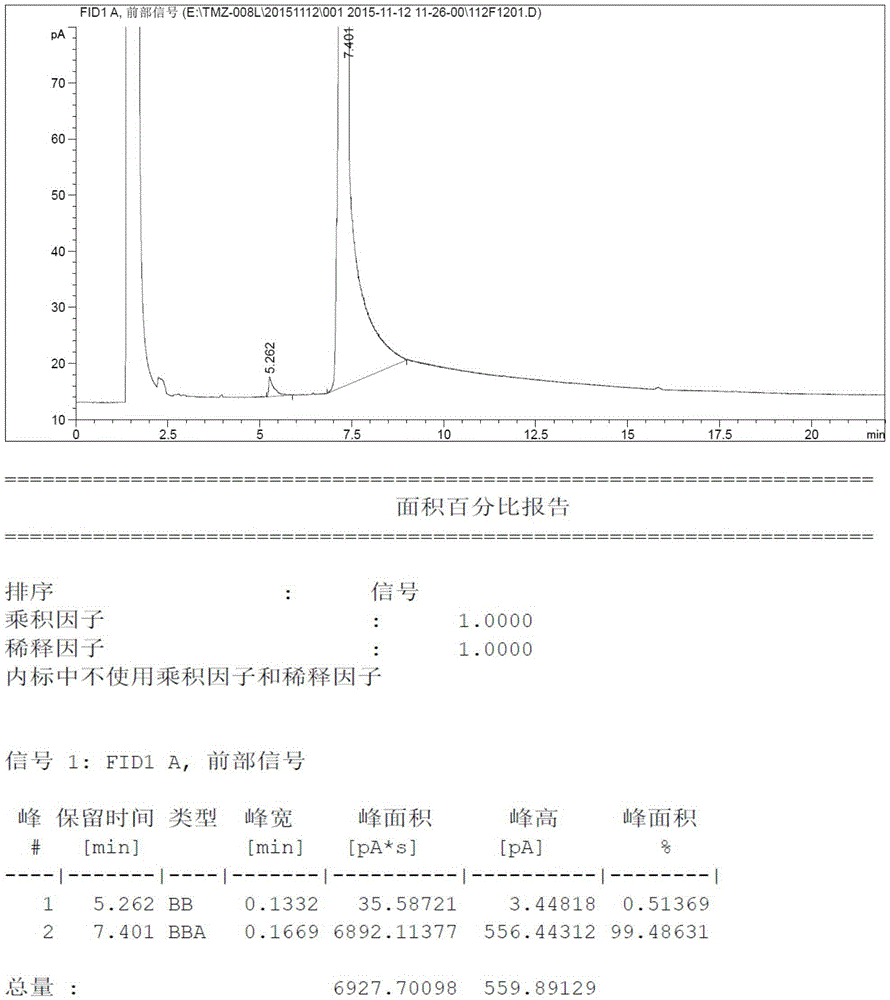
[0030] Add 8.21 grams (100mmoL) of N-methylimidazole and 80ml tetrahydrofuran into a three-necked reaction flask, then drop the temperature to -15°C and add 48ml of n-butyllithium-n-hexane solution (containing n-butyllithium 120mmoL, 2.5mol / L), after the dropwise addition was completed, it was incubated and reacted at -15°C for 2 hours, then 3.84 grams (120mmoL) of elemental sulfur was added in batches at this temperature, and after the addition was completed, it was slowly raised to room temperature and stirred for 10 hours. Thin-layer chromatography detected no raw material spots, then quenched in an ice-water bath, added dilute hydrochloric acid to adjust the pH to 5.0-5.5, and then concentrated the reaction system to dryness under pressure. Then the concentrated solid was added to ethyl acetate and activated carbon for heating and dissolving, refluxed for decolorization for about 1 hour, filtered, and the filtrate was concentrated under reduced pressure to obtain 9.04 gram...
Embodiment 2
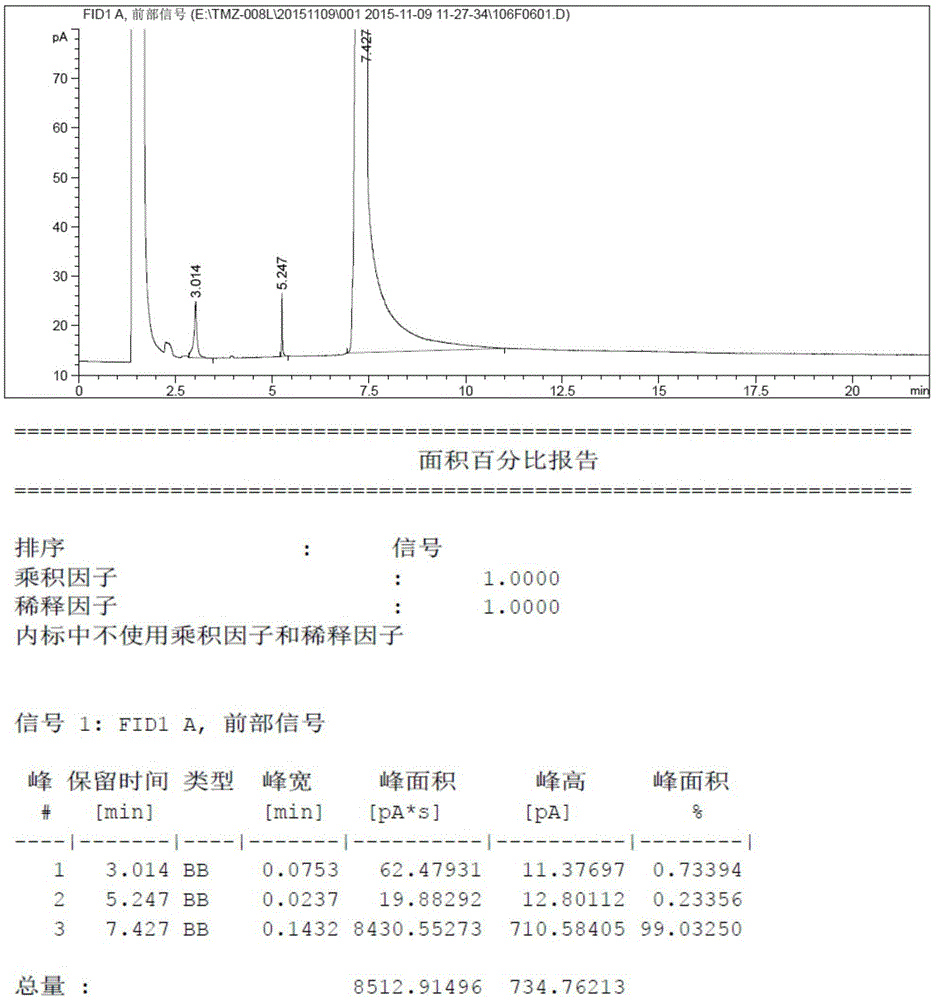
[0032] Add 8.21 grams (100mmoL) of N-methylimidazole and 80ml tetrahydrofuran into a three-necked reaction flask, then drop the temperature to -10°C and add 48ml of n-butyllithium-n-hexane solution (containing n-butyllithium 120mmoL, 2.5mol / L), after the dropwise addition was completed, it was incubated and reacted at -10°C for 1.5 hours, then 4.48 grams (140mmoL) of elemental sulfur was added in batches at this temperature, and the temperature was raised to reflux for 9 hours after the addition was completed. Thin-layer chromatography detected no raw material spots, then quenched in an ice-water bath, added dilute hydrochloric acid to adjust the pH to 5.5-6.0, and then concentrated the reaction system to dryness under pressure. Then the concentrated solid was added to ethyl acetate and activated carbon for heating and dissolving, refluxed for decolorization for about 1 hour, filtered, and the filtrate was concentrated under reduced pressure to obtain 9.52 grams of light yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com