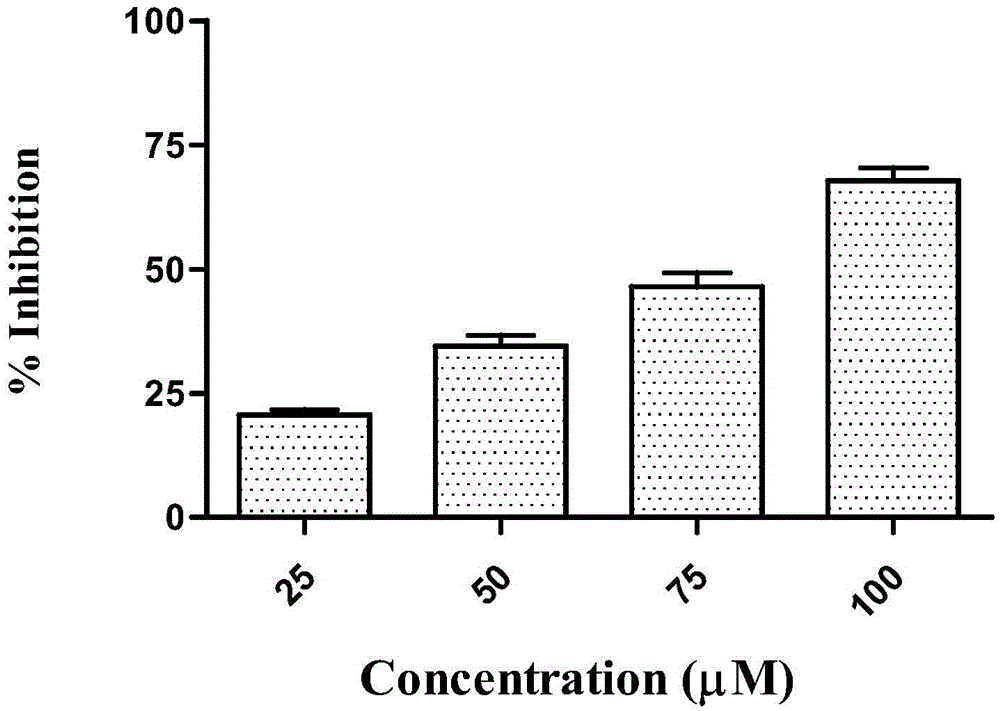
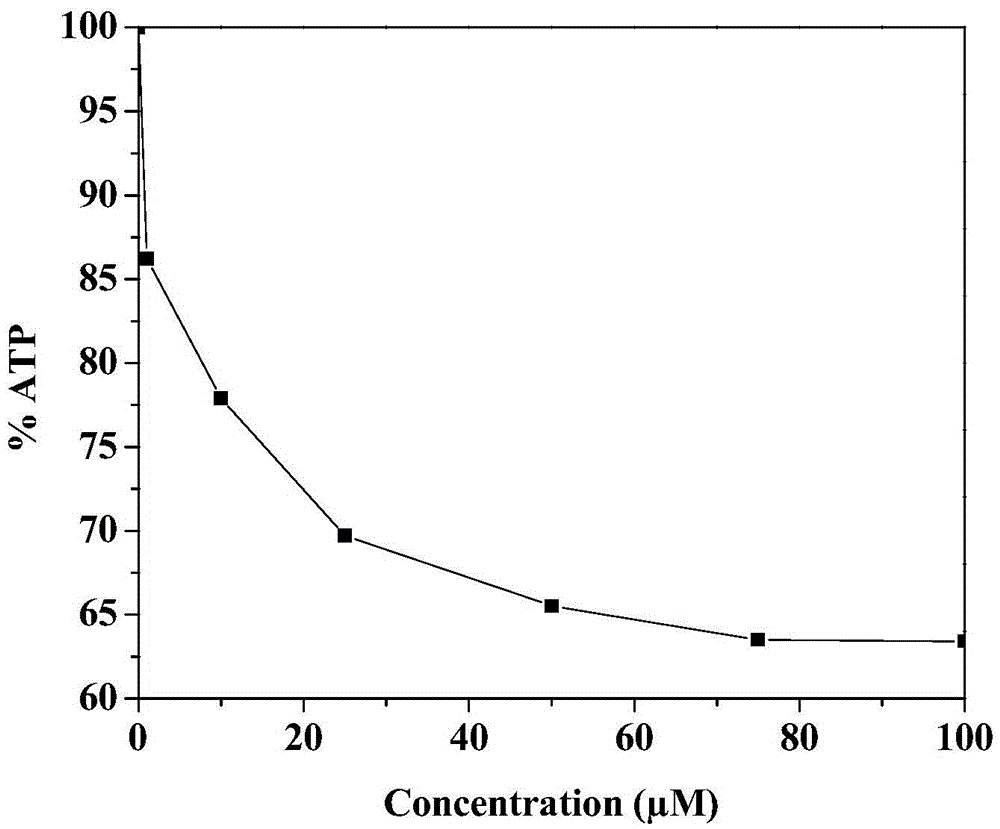
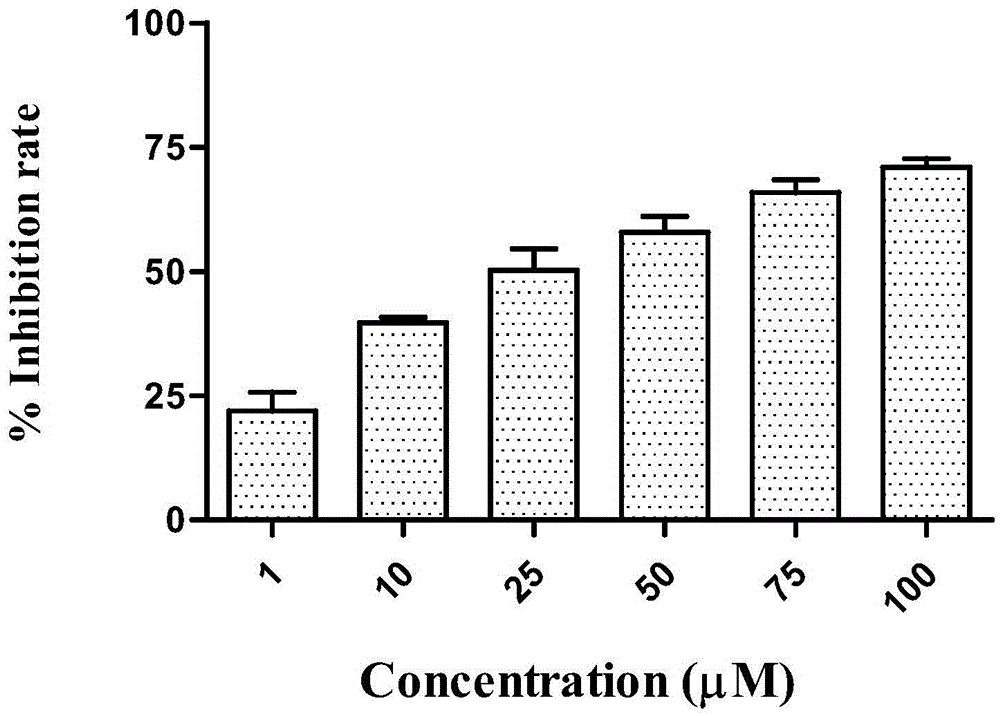
Anti-tumor drug compound, preparation method and application
A technology of anti-tumor drugs and compounds, applied in the field of energy metabolism that inhibits anaerobic glycolysis process, can solve the problem of limited tumor suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 (best synthesis example)
[0030] 2-Deoxyglucose (78mg, 0.48mmol): Metformin hydrochloride (80mg, 0.48mmol), dissolved in 20mL methanol, nitrogen gas for 0.5h, 40°C for 3h, naturally cooled to room temperature after the reaction, and distilled off the solvent under reduced pressure , recrystallized from ethanol-ether (v / v-1:1), and dried in vacuo to obtain 140 mg of the product.
[0031] Molecular formula C 10 h 23 N 5 o 5 Elemental analysis: C, 40.83; H, 7.85; N, 23.75%. Ultraviolet UV-vis (CH 3 OH / nm)(ε×10 -4 ,dm 3 mol -1 cm -1 ):232(3.4).Infrared Spectrum IR(KBr,ν / cm -1 ):3371m, 3160m, 2900-2995m, 1628s, 1556s, 1468s, 1078s, 1007s, 710m, .600cm -1.
Embodiment 2
[0033] 2-deoxyglucose (248mg, 1.44mmol): metformin hydrochloride (80mg, 0.48mmol), wherein the molar ratio of 2-deoxyglucose to metformin hydrochloride is 3:1, dissolved in 10mL of methanol (the amount of 2-deoxyglucose substance and methanol The volume ratio is 1.44mmol: 10ml), blow nitrogen for 0.5h, and react at 10°C for 1h. After drying, 40 mg of the product was obtained, indicating that the yield of this synthetic scheme was lower than that of the best practice case.
Embodiment 3
[0035] 2-deoxyglucose (78mg, 0.48mmol): metformin hydrochloride (254mg, 1.44mmol), wherein the molar ratio of 2-deoxyglucose to metformin hydrochloride is 1:3, dissolved in 40mL of methanol (the amount of 2-deoxyglucose substance and methanol The volume ratio is 0.48mmol: 40ml), blowing nitrogen for 0.5h, reacting at 80°C for 6h, naturally cooling to room temperature after the reaction, distilling off the solvent under reduced pressure, recrystallizing from ethanol-ether (v / v-1:1), After vacuum drying, 70 mg of the product was obtained, which indicated that the yield of this synthetic scheme was lower than that of the best implementation case.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com