Pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, to achieve the effects of enhancing memory, convenient administration, and small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] In a specific embodiment, the preparation method of the pharmaceutical composition comprises the following steps:
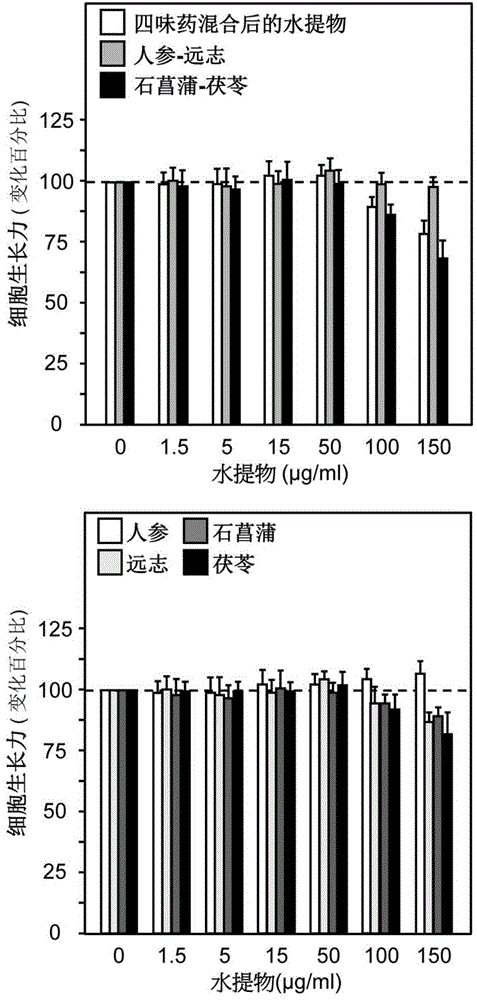
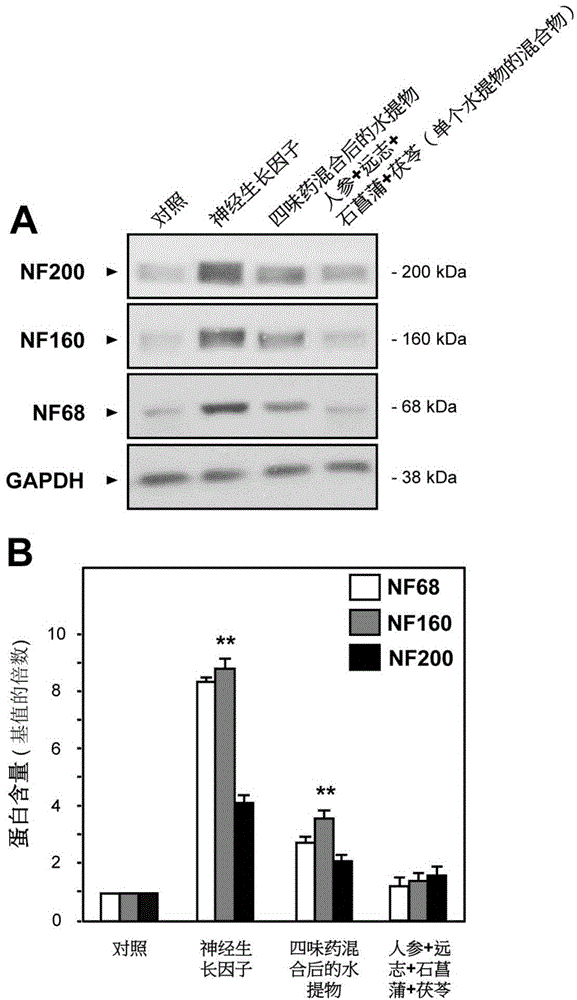
[0054] Take ginseng, Polygala, Shichangpu, and Poria and cut them into pieces; weigh ginseng-polygala (weight ratio 1:1) and Shichangpu-Poria (weight ratio 1:2), and soak them in 6-10 times of water for 20-40 Minutes, extract 1-3 times, 1-3 hours each time, combine extracts, freeze-dry to obtain water extract powder. Combine the water extract of ginseng-Polygalae medicine pair and Shichangpu-Poria cocos medicine pair water extract in a weight ratio of 1:5, and mix well.
[0055] In a more specific embodiment, ginseng, Polygala, Shichangpu and Poria are respectively cut into pieces. Take ginseng and polygala (weight ratio 1:1), put them in a 500mL conical flask, add 120-200g of water, soak for 20-40 minutes, heat and extract for 1-3 hours, filter while hot, and heat the residue as above Extract 1-2 times and combine the filtrates. The filtrate was lyophi...
Embodiment 1
[0068] Embodiment 1: the preparation of compound medicine combination
[0069] Take respectively 10 g of ginseng (dried root of Araliaceae plant ginseng (Panaxginseng C.A.Mey.), Polygala (dried root of Polygalate nuifolia Willd. or Polygalasibirica L.), 10 g of Polygala (Panaxginseng C.A. Mey.), 10g of dried rhizome of Araceae plant Acorus tarinowii Schott), 20g of Poria cocos (dried sclerotia of Polyporaceae fungus Poriacocos (Schw.) Wolf) and cut into pieces. Take ginseng and polygala, put them in a 500mL Erlenmeyer flask, add 160g of water, soak for 30 minutes, heat and extract for 2 hours, filter while hot, heat and extract the residue once according to the above method, and combine the filtrates. The filtrate was lyophilized to obtain the aqueous extract lyophilized powder I. Then take the two flavors of Acorus calamus and Poria, put them in a 500mL Erlenmeyer flask, add 240g of water, soak for 30 minutes, heat and extract for 2 hours, filter while hot, heat and extract ...
Embodiment 2
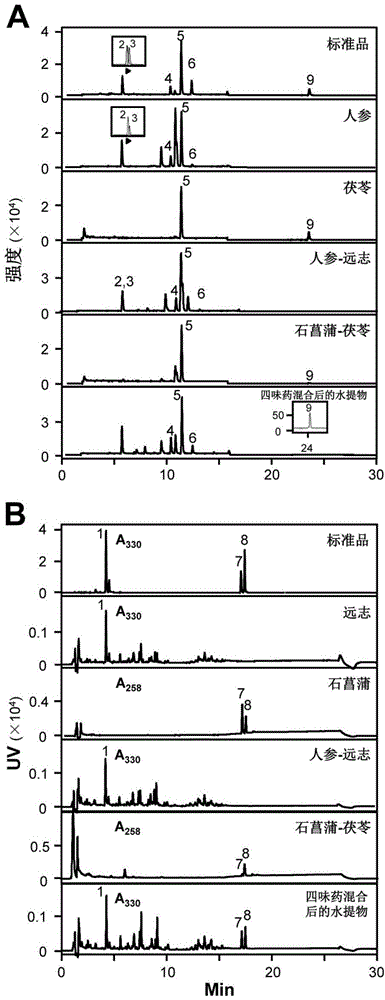
[0070] Embodiment 2: the quality control of ginseng-polygala medicine pair and Shichangpu-Poria cocos medicine pair
[0071] Based on the research on the chemical constituents and functions of ginseng, Polygalaceae, Shichangpu and Poria cocos, select ginsenoside Rb 1 , Rd, Re, Rg 1 , Polygala 3,6-di-mustardyl sucrose, Acorus α-asarone and β-asarone and Poria cocos as index components.
[0072] Accurately weigh 1 mg of the water extract freeze-dried powder I and II of Example 1, the single drug water extract freeze-dried powder and the water extract freeze-dried powder mixed with the four drugs, add it to 5 mL of 50% ethanol solution, and ultrasonicate for 30 Minutes, through a 220μM filter membrane, that is, the test product.
[0073] The test substance was determined by LC-MS system (liquid-mass spectrometry system). HPLC system (Agilent1290, binary pump, autosampler, DAD detector, column thermostat), C18 reverse column (Agilent ZORBAX Eclipse XDB-C18column (1.8mm, 50mm×4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com