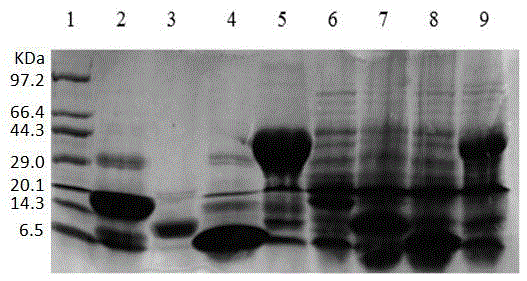
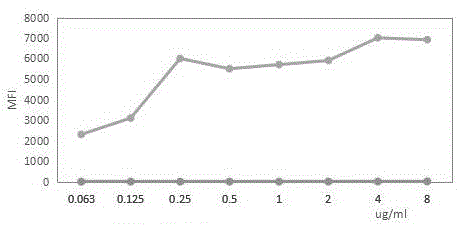
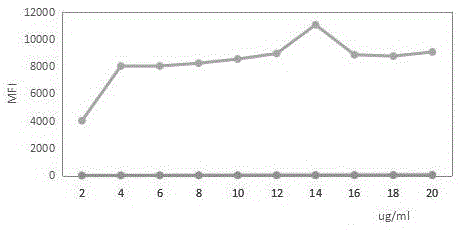
A liquid-chip HIV-antigen antibody combined detection kit and a detecting method
An antigen-antibody, combined detection technology, applied in the field of immunoassay, can solve the problem of high false positive rate, and achieve the effect of ensuring blood transfusion safety, early detection, and improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219]
[0220]105 specimens from Tianjin Blood Center were analyzed, luminexxMAP detected 8 positive specimens in 19 specimens with indeterminate WB but positive RNA, and 3 negative specimens were detected in luminexxMAP in 18 specimens with indeterminate WB but negative RNA Specimens, indicating that luminexxMAP can reduce the occurrence of indeterminate specimens to a certain extent. Among the 3 WB-negative but RNA-positive samples, the detection result of luminexxMAPHIV-1 antibody in 1 case was indeterminate, which avoided the missed detection of WB to a certain extent, indicating that the specificity of luminexxMAP was higher than that of WB. The sensitivity of LuminexxMAP detection was 82.7% (72 / 87) higher than that of WB 74.7% (65 / 87).
Embodiment 2
[0222]
[0223] Analysis of the test results of 33 cases of RNA-positive gay men and women showed that 13 cases were positive for HIV-1 antibody by WB, 18 cases were indeterminate samples for HIV-1 antibody, and 2 cases were negative for HIV-1 antibody; 19 cases were positive for luminexxMAP. Uncertain specimens were 14 cases. By comparison, it was found that the detection sensitivity of luminexxMAP was 57.8% (19 / 22) higher than that of WB (39.4%).
Embodiment 3
[0225]
[0226] HIV immunoblotting test judgment criteria have always been based on antibody detection: the formation of at least two Env bands (gp41, gp120 / gp160) or one Env band plus p24 band is judged as positive, and the band type does not meet the positive judgment criteria. The result is judged as positive Suspicious, no HIV banding results are judged as negative, which can identify false positive results of the primary screening test. image 3 The results show that the detection of luminexxMAP antigen can suggest to a certain extent that the specimen may be before or during the positive conversion of the antibody, but the HIV-1p24 antigen is transiently increased in the infected person, so two cases of HIV-1, M1126 and M1127, also appeared Antibody positive, HIV-1p24 antigen negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com