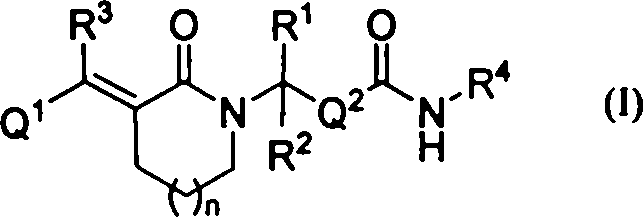
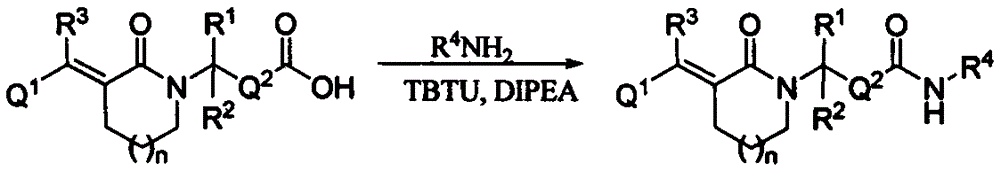
Lactam histone deacetylase inhibitors
A compound, selected technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] (E)-N-(2-aminophenyl)-4-((2-oxo-3-(pyridin-2-ylmethylene)piperidin-1-yl)methyl)benzamide
[0089]
[0090] Step a: Preparation of tert-butyl-2-oxopiperidine-1-carboxylic acid
[0091] Add piperidin-2-one (5g, 50mmol), di-tert-butyl dicarbonate (13.1g, 60mmol) and acetonitrile 100mL in sequence in a 250mL eggplant-shaped flask, cool to 0°C in an ice bath, and slowly add 4-bis Aminopyridine (1.22g, 10mmol, after addition, reacted at room temperature for 24h, TLC detected that the reaction was complete, evaporated the solvent under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 15: 1) to obtain Color oily liquid (6.07g, 30.5mmol). 1 H-NMR (300MHz, CDCl 3 )δ3.65(m, 2H), 2.50(m, 2H), 1.82(m, 4H), 1.52(s, 9H).ESIMS: [M+H] + : 222.12.
[0092] Step b: (E)-tert-Butyl-2-oxo-3-(pyridin-2-ylmethylene)piperidine-1-carboxylic acid tert-butyl ester
[0093]Add tert-butyl-2-oxopiperidine-1-carboxylic acid (...
Embodiment 2
[0103] (E)-N-(2-amino-4-fluorophenyl)-4-[[2-oxo-3-(pyridin-2-ylmethylene)piperidin-1-yl]methyl]benzene Formamide
[0104]
[0105] Using piperidin-2-one, 4-fluoro-1,2-phenylenediamine as raw materials, pyridine-2-carbaldehyde, and methyl p-bromomethylbenzoate, the target compound white was obtained by a method similar to Example 1 Solid (E)-N-(2-amino-4-fluorophenyl)-4-[[2-oxo-3-(pyridin-2-ylmethylene)piperidin-1-yl]methyl] Benzamide, yield 41.1%; Mp: 217-219°C; 1 H-NMR (300MHz, DMSO-d6) δ9.60(s, 1H), 8.67(d, J=3.9Hz, 1H), 7.96(d, J=8Hz, 1H), 7.87-7.81(m, 1H) , 7.63(s, 1H), 7.55(d, J=7.9Hz, 1H), 7.40(d, J=8.1Hz, 2H), 7.34-7.30(m, 1H), 7.13-7.08(m, 1H), 6.53(dd, J=11.3, 3Hz, 1H), 6.39-6.32(m, 1H), 5.25(s, 2H), 4.73(s, 2H), 3.4(s, 2H), 3.18-3.15(m, 2H ), 1.82-1.80 (m, 2H).ESIMS: [M+Na] + : 453.2.
Embodiment 3
[0107] (E)-N-(2-Aminophenyl-4-[[3-(furan-2-ylmethylene)-2-oxopiperidin-1-yl]methyl]benzamide
[0108]
[0109] The preparation method was as in Example 1 to obtain a white solid with a yield of 62.3%. Mp: 212-214°C; 1 H-NMR (300MHz, DMSO-d 6 )δ9.66(s, 1H, -CONHAr-), 7.95(d, J=8Hz, 2H, H-Ar), 7.84(d, J=8Hz, 2H, H-Ar), 7.84-7.83(m, 1H, H-Ar), 7.62 (s, 1H, -CH=), 7.4 (d, J=1Hz, 2H, H-Ar), 7.12-7.08 (m, 1H, H-Ar), 6.80 (d, J=3.3Hz, 1H, H-Ar), 6.66-6.64(m, 1H, H-Ar), 6.53(dd, J 1 =11Hz,J 2 =3Hz, 1H, H-Ar), 6.39-6.32(s, 1H, H-Ar), 5.24(s, 2H, -NH 2 ), 4.65 (s, 2H, -CH 2 -), 2.88(s, 2H, -CH 2 -), 1.84(s, 2H, -CH 2 -), 1.24(m, 2H, -CH 2 -);IR(KBrcm -1 ): 3457, 3348, 3292, 3104, 2989, 2949, 2389, 1728; MSm / z: 424.2 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com