A kind of thioxanthone dicarboxamide compound and its preparation method and application
A thioxanthone dicarboxamide and xanthone dicarboxamide technology are applied in the field of thioxanthone dicarboxamide compounds and their preparation, achieving high photo-initiating activity, good ultraviolet absorption capacity, and improving photo-initiating efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: 1,2-thioxanthone diamide carboxylic acid (TX-COOH)
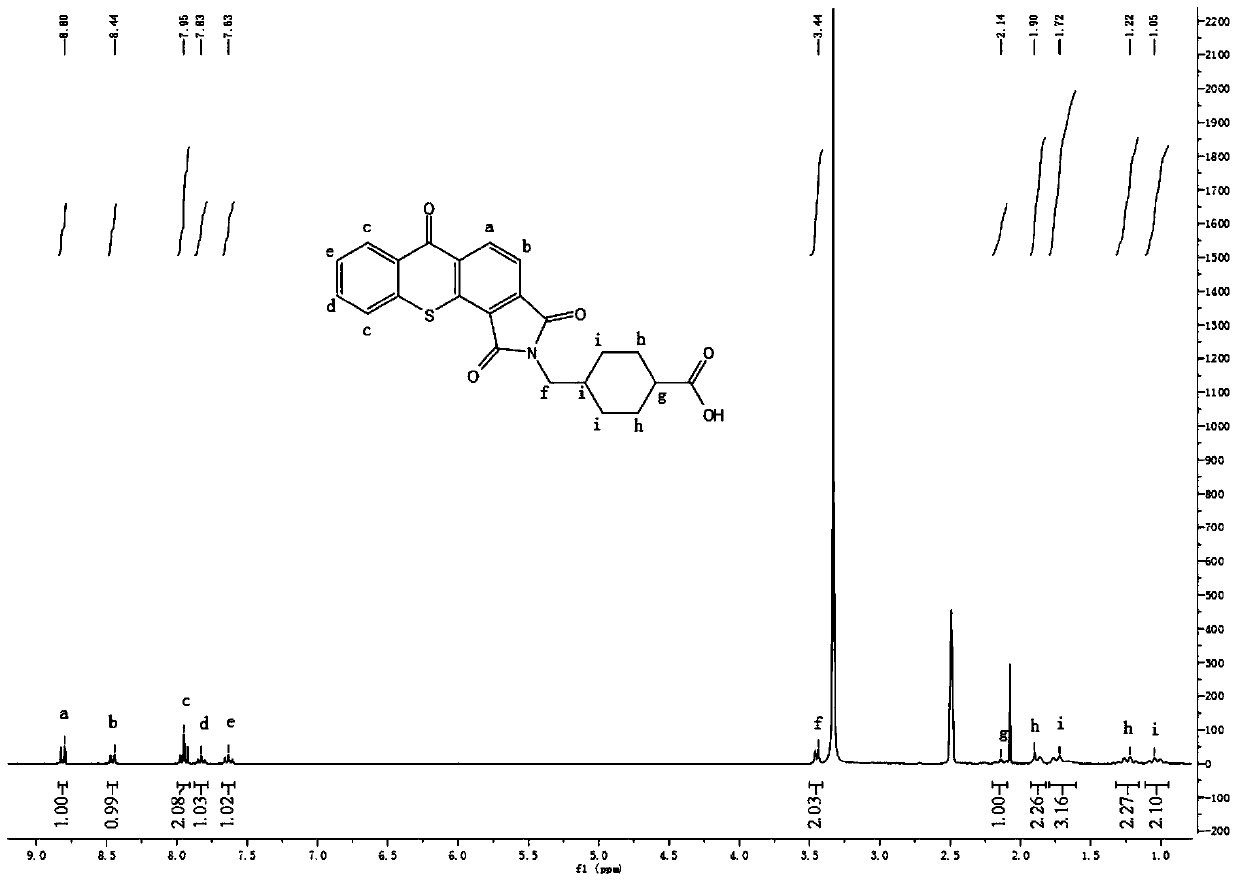
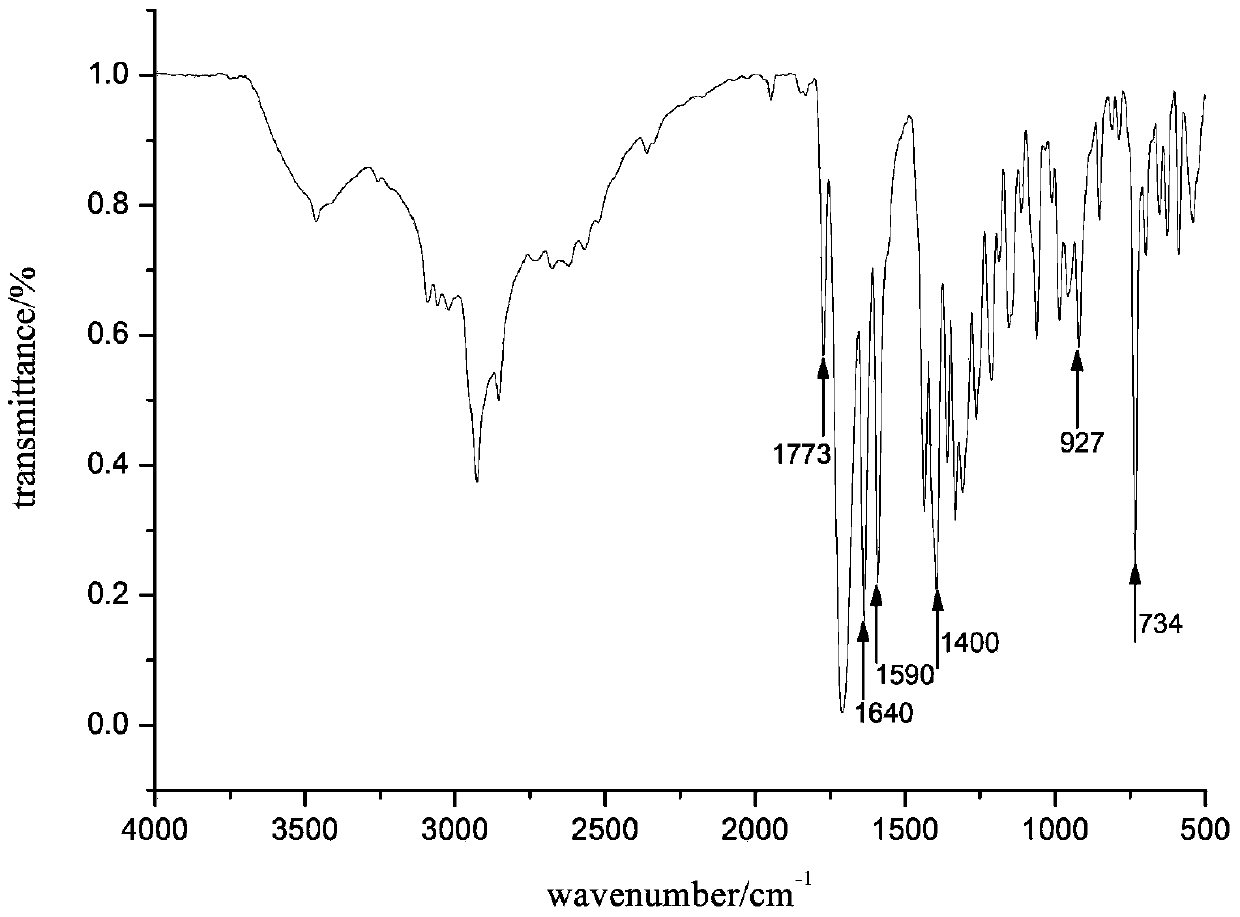
[0050] Take a 250ml round bottom flask, add 1,2-thioxanthone diacid anhydride (0.4760g, 1.69mmol), dissolve it in 220ml glacial acetic acid under heating, add 0.2651g (1.69mmol) tranexamic acid, and react at 123°C under reflux for 3h. After cooling to room temperature, standing overnight to precipitate a precipitate, filtering, washing with xylene, and drying in vacuum to obtain a yellow crystal product with a yield of 66.1%. 1 H NMR(300MHz, DMSO-d 6 ): δ 1.05 (m, 2H), 1.23 (m, 2H), 1.73 (m, 3H), 1.90 (m, 2H), 2.14 (m, 1H), 3.44 (d, 2H), 7.63 (t, 1H),7.82(t,1H),7.95(t,2H),8.44(d,1H),8.80(d,1H).IR(KBr,cm -1 ):734(υ C-S ),927,1400(υ O=H ),1590(υ C=C ),1640,1773(υ C=O ).Anal.Calcd.for C 23 H 19 NO 5 S: C, 65.54; H, 4.54; N, 3.32; S, 7.61%; Found: C, 64.34; H, 4.59; N, 3.27; S, 7.46.
[0051] Product 1 H NMR and FT-IR spectra are as figure 1 with figure 2 Shown.
Embodiment 2
[0052] Example 2: 1,2-thioxanthone diamide ethanol (TX-OH)
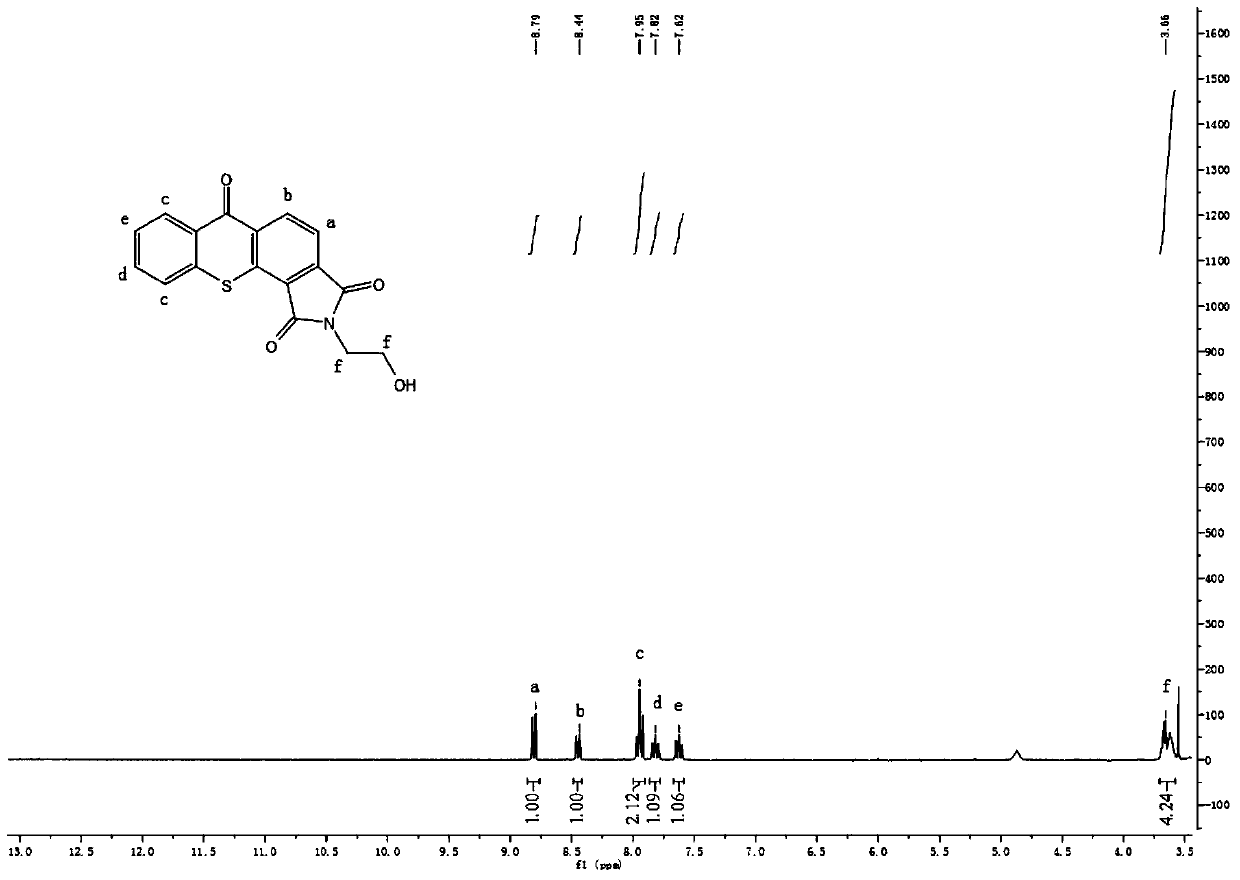
[0053] Add 0.423g of 1,2-thioxanthone diacid anhydride and 50ml of dioxane to a 150ml round bottom flask, stir to dissolve the reactants completely, add 20ml of 0.092g ethanolamine in dioxane solution, and react at 102°C for 3h. After cooling to room temperature, the solvent was removed by rotary evaporation, and then fully washed with ether to obtain a yellow product with a yield of 94%. 1 H NMR (DMSO): δ=8.79 (1H, d, Ha), 8.44 (1H, d, Hb), 7.95 (2H, t, Hc), 7.82 (1H, t, Hd), 7.62 (1H, t, He),3.66(4H,m,Hf).IR(KBr,cm -1 ):735(υC-S),1589(υ C=C ),1640,1765(υ C=O ),1463(υ O=H ).Anal.Calcd.forC 17 H 11 NO 4 S: C, 62.76; H, 3.41; N, 4.31; S, 9.85%; Found: C, 62.59; H, 3.49; N, 4.33; S, 9.91.
[0054] Product 1 H NMR and FT-IR spectra are as image 3 with Figure 4 Shown.
Embodiment 3
[0055] Example 3: 1,2-thioxanthone diamide thiol (TX-SH)
[0056] Add 0.282g of 1,2-thioxanthone diacid anhydride and 100ml of glacial acetic acid to a 150ml round bottom flask, heat to completely dissolve the reactants, add 0.077g mercaptoethylamine, reflux for 3h at 123°C, cool to room temperature, and let stand overnight The precipitate was separated out, filtered, washed with xylene, and dried under vacuum at 60°C to obtain a yellow crystal product with a yield of 76.2%. 1 H NMR (DMSO): δ = 8.85 (1H, d, Ha), 8.48 (1H, d, Hb), 7.99 (2H, t, Hc), 7.85 (1H, t, Hd), 7.66 (1H, t, He), 3.79(2H,m,Hf),2.78(2H,m,Hg).IR(KBr,cm -1 ):736(υ C-S ),1583(υ C=C ),1640,1767(υ C=O ),2537(υ S-H ).Anal.Calcd.for C 17 H 11 NO 3 S 2 : C, 59.81; H, 3.25; N, 4.10; S, 18.78%; Found: C, 59.72; H, 3.26; N, 4.01; S, 18.40.
[0057] Product 1 H NMR and FT-IR spectra are as Figure 5 with Image 6 Shown.
[0058] The ultraviolet-visible absorption spectra of the three thioxanthone dimethylformamide deriva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com