Synthesis method for benzothiazole unit-based covalent organic framework material
A technology of covalent organic framework and benzothiazole is applied in the field of synthesis of covalent organic framework materials, which can solve the problems of difficult functionalization and harsh synthesis conditions of carbon nitride materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
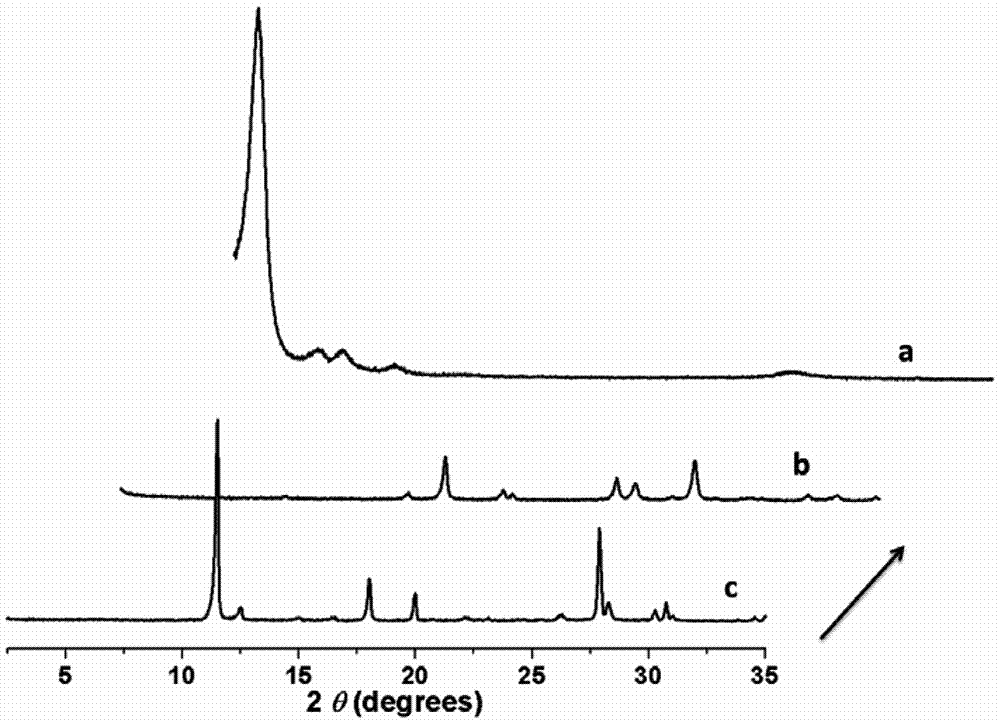
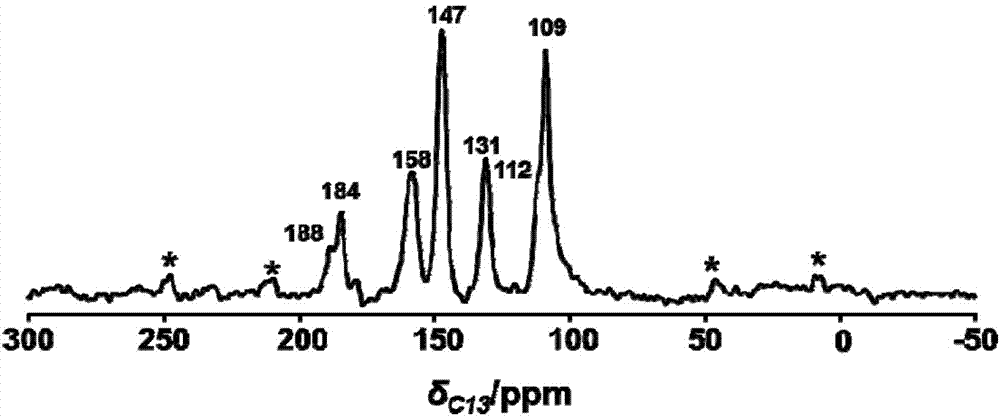
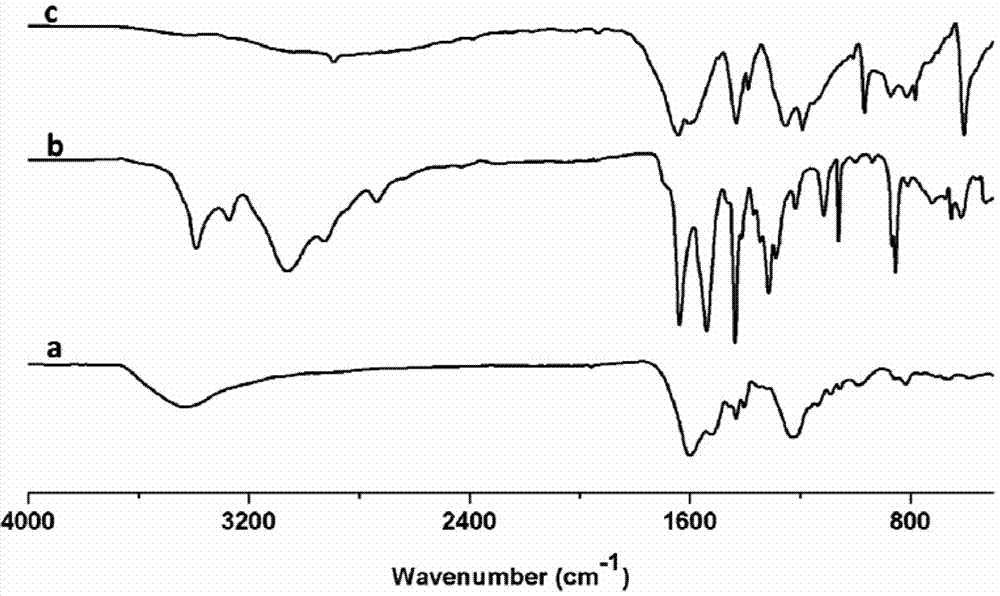
[0028] Add 10.5 mg of 2,4,6-trihydroxy-triphenylcarbaldehyde (1) and 16.7 mg of 2,6-diaminobenzobithiazole (2) into a pressure-resistant glass tube, then add 0.7 mL of dioxane and 0.3 Add 0.2 mL of 3 mol / L acetic acid aqueous solution to 0.2 mL of tetrahydrofuran, shake well, freeze the glass tube through a liquid nitrogen bath, vacuumize it, seal the tube with a flame, and raise the temperature to 120°C for 3 days after sealing the tube. After the reaction was completed, the glass tube was opened, quenched with acetone, centrifuged, and the solid product was washed three times with acetone and tetrahydrofuran, and then dried at 100°C to obtain 21 mg of brown-red solid powder (named COF-LZU80), with a yield of 86 %, the reaction is as follows:
[0029] .
Embodiment 2
[0031] Add 10.5mg of 2,4,6-trihydroxy-trithenylaldehyde and 16.7mg of 2,6-diaminobenzobithiazole into the autoclave, then add 1.0mL of 1-butyl-3-methylimidazolium tetrafluoroboric acid salt, shake well, add 0.2 mL of 3 mol / L acetic acid aqueous solution, close the kettle, and raise the temperature to 120°C for 3 days. After the reaction, the autoclave was opened, quenched with acetone, centrifuged, and the solid product was washed three times with acetone and tetrahydrofuran, and dried at 100°C to obtain a brown-red solid powder (named COF-LZU80).
Embodiment 3
[0033] Add 10.5mg of 2,4,6-trihydroxy-triphenylcarbaldehyde and 16.7mg of 2,6-diaminobenzodithiazole into a pressure-resistant glass tube, then add 0.7mL of dioxane and 0.3mL of tetrahydrofuran, shake well Add 0.2 mL of water. After the glass tube was frozen in a liquid nitrogen bath, the tube was evacuated, and then the tube was sealed with a flame. After the tube was sealed, the temperature was raised to 120° C. for 3 days. After the reaction, the glass tube was opened, quenched with acetone, centrifuged, and the solid product was washed three times with acetone and tetrahydrofuran, and then dried at 100°C to obtain a brown-red solid powder (named COF-LZU80).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com