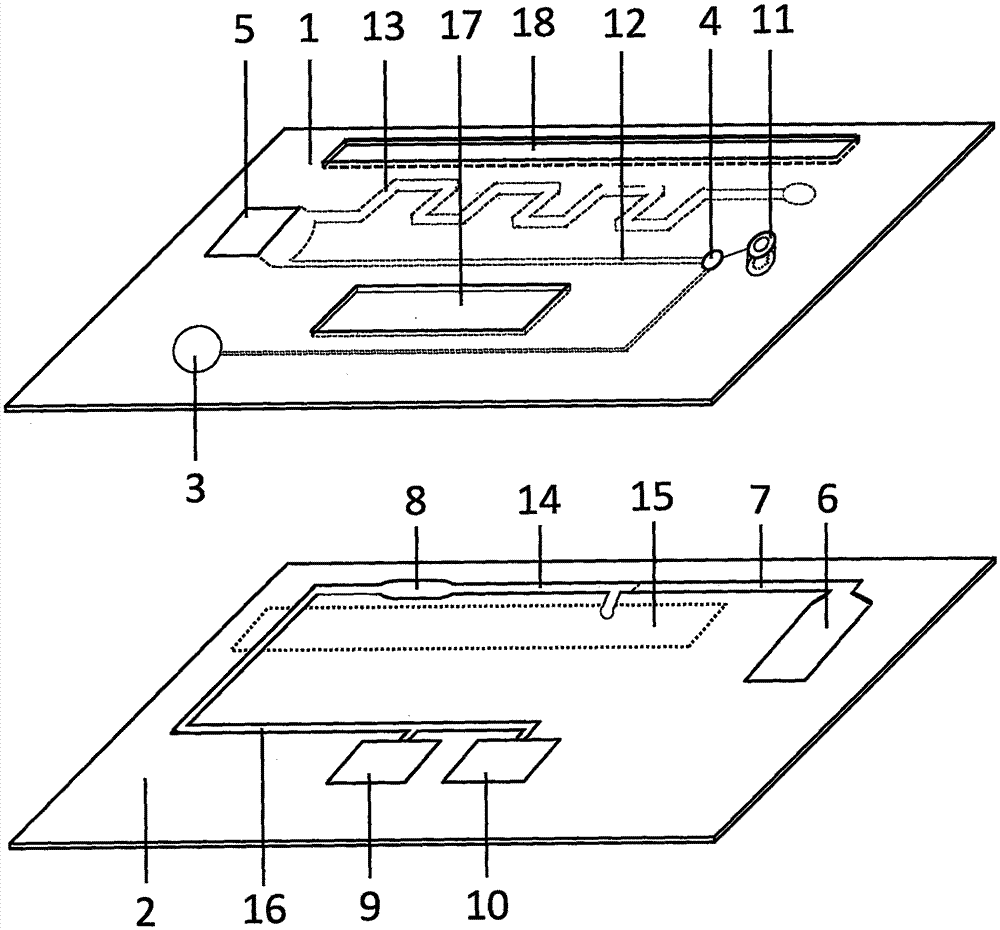
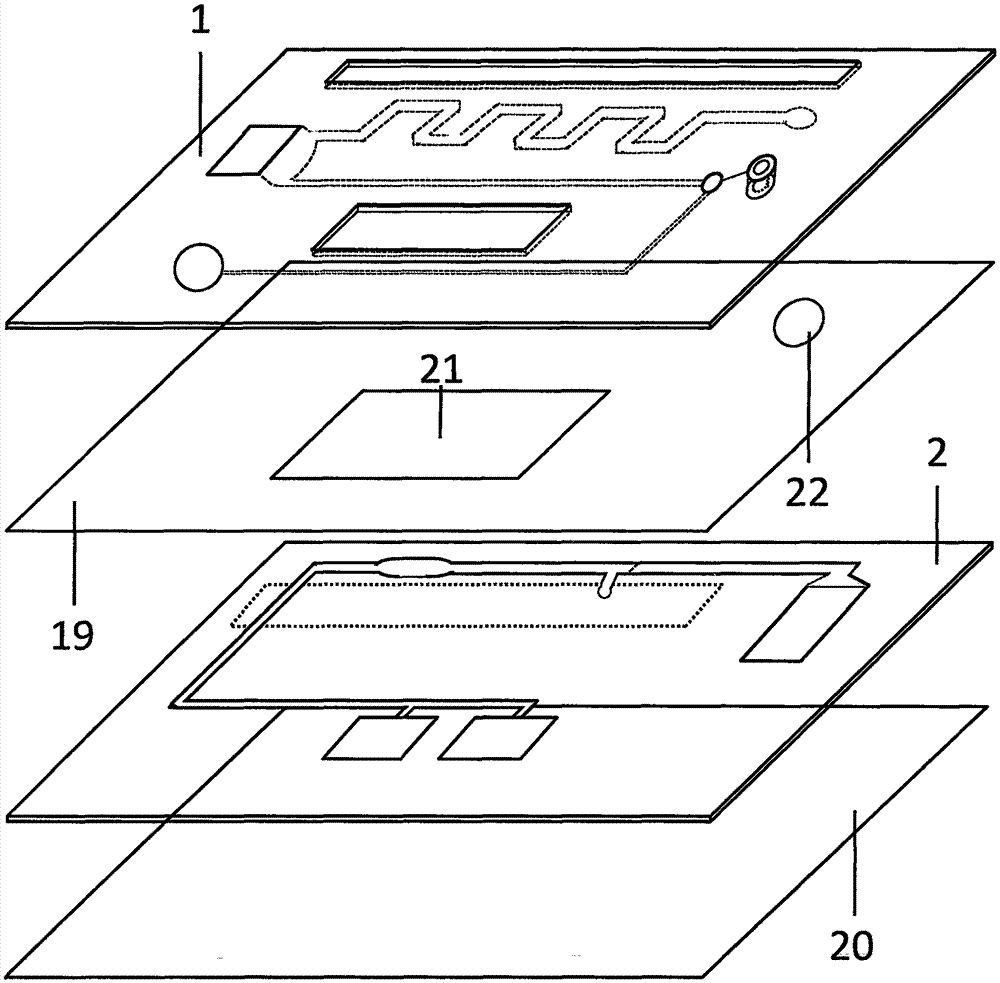
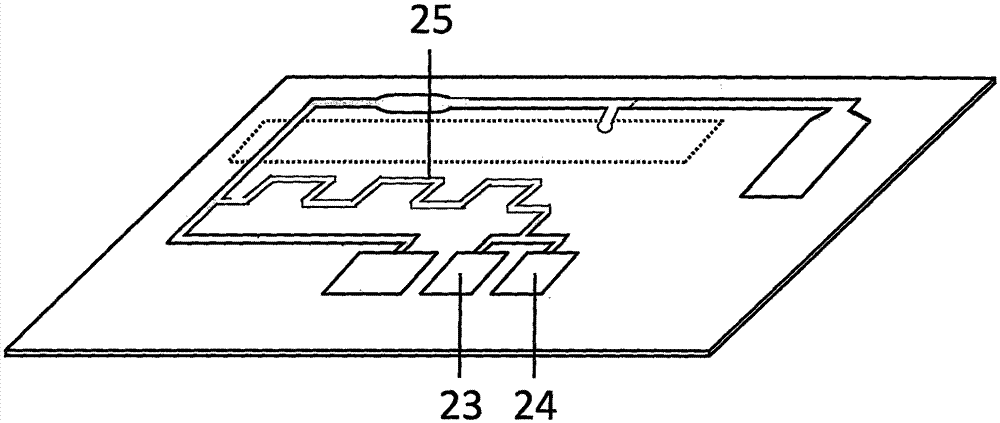
Magnetic particle chemiluminescent microfluidic chip for quantitatively detecting troponin I in whole blood
A microfluidic chip, quantitative detection technology, applied in chemical instruments and methods, laboratory containers, laboratory utensils, etc., can solve the problems of interference, expensive instruments, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Enzymatic chemiluminescent assay of cTnI
[0062] (1) Antibody labeling
[0063] Dissolve 50 μg of HRP in 1 mL of distilled water, then add 10 μmol of fresh NaIO 4 The solution was reacted at room temperature in the dark for 20 minutes, and the purified solution was dialyzed against 1 mM pH 4.4 sodium acetate buffer. Then adjust the pH to 9.0 with pH 9.5 carbonate buffer, add 100 μg of anti-cTnI monoclonal antibody, and react at room temperature for 2 h in the dark. Add 0.1mL4mg / mL new NaBH 4 , Mixed and reacted at 4°C for 2h. Put the above solution into a dialysis bag, dialyze against 0.15MpH7.4PBS, overnight at 4°C, and obtain HRP-labeled cTnI antibody.
[0064]Add 1 mg magnetic particles (diameter 2 μm), 10 μg EDC and 15 μg NHS solution and 10-30 μg anti-cTnI monoclonal antibody (different from HRP-labeled antibody) solution to pH 7.4 phosphate buffer, mix well and react at room temperature for 4 h, add 1 mg glycine blocked. Enrichment and purificati...
Embodiment 2
[0075] Example 2: Direct chemiluminescent assay of cTnI
[0076] (1) Antibody labeling
[0077] Add an appropriate amount of activated acridinium ester and 100 μg anti-cTnI monoclonal antibody solution to the phosphate buffer, mix well, react at room temperature for 3 hours, and add 1 mg glycine to block. Separation and purification by dialysis to obtain the acridinium ester-labeled cTnI antibody.
[0078] Add 1 mg of magnetic particles (1 μm in diameter), 20 μg of EDC, 20 μg of NHS solution and 30 μg of streptavidin to 1 ml of 10 mM pH7.4 phosphate buffer, mix well and react at room temperature for 4 h, add 1 mg of glycine to block. Enrichment by magnet adsorption to remove unreacted streptavidin to obtain magnetic particle-labeled streptavidin.
[0079] Add 20 μg of anti-cTnI monoclonal antibody to 10 μL of 0.25 mg / mL Sulfo-NHS-LC-biotin solution, and react for 1 h. Purify by ultrafiltration and centrifugation to remove unreacted biotin to obtain biotin-cTnI antibody.
...
Embodiment 3
[0090] Example 3: Magnetic Particle Size Screening
[0091] Refer to Example 2 for other experimental conditions, and the magnetic particle size and magnetic induction of the magnet are carried out according to the following scheme.
[0092] The particle size is 0.1 μm, 0.5 μm, 1.0 μm, 2.0 μm, 2.4 μm, 3 μm, 10 μm. The magnetic induction of the magnet is 500 Gauss, 1000 Gauss, 4000 Gauss, 8000 Gauss, 12000 Gauss, 30000 Gauss. Magnetic particles of seven sizes are respectively driven by the six kinds of magnets.
[0093] The experimental results show that when the 0.1μm magnetic particles are combined with the 500 Gauss magnet, the minimum detection limit is 500pg / ml, the quantitative detection range is 0.5~50ng / ml, and the linear correlation coefficient R 2 >0.95; intra-assay and inter-assay repeatability are less than 20%. That is: the chemiluminescent signal is weak, the sensitivity is not high, and the repeatability is poor.
[0094] When 10μm magnetic particles and 30,0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com