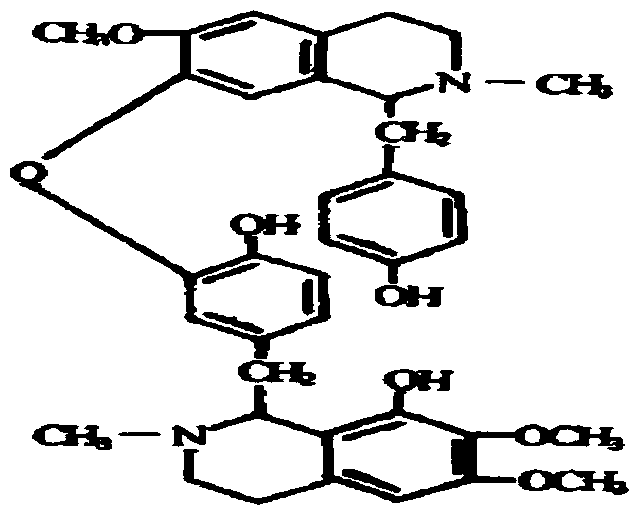
Liansinline suppository and its preparation method and application
A liensinine suppository and the technology of liensinine are applied in the directions of suppository delivery, pharmaceutical formulation, cardiovascular system diseases, etc., and can solve the problems of no commercial listing of liensinine, the inability to achieve the expected ideal pharmacological activity, and serious liver first-pass effect, etc. , to avoid the effect of poor oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
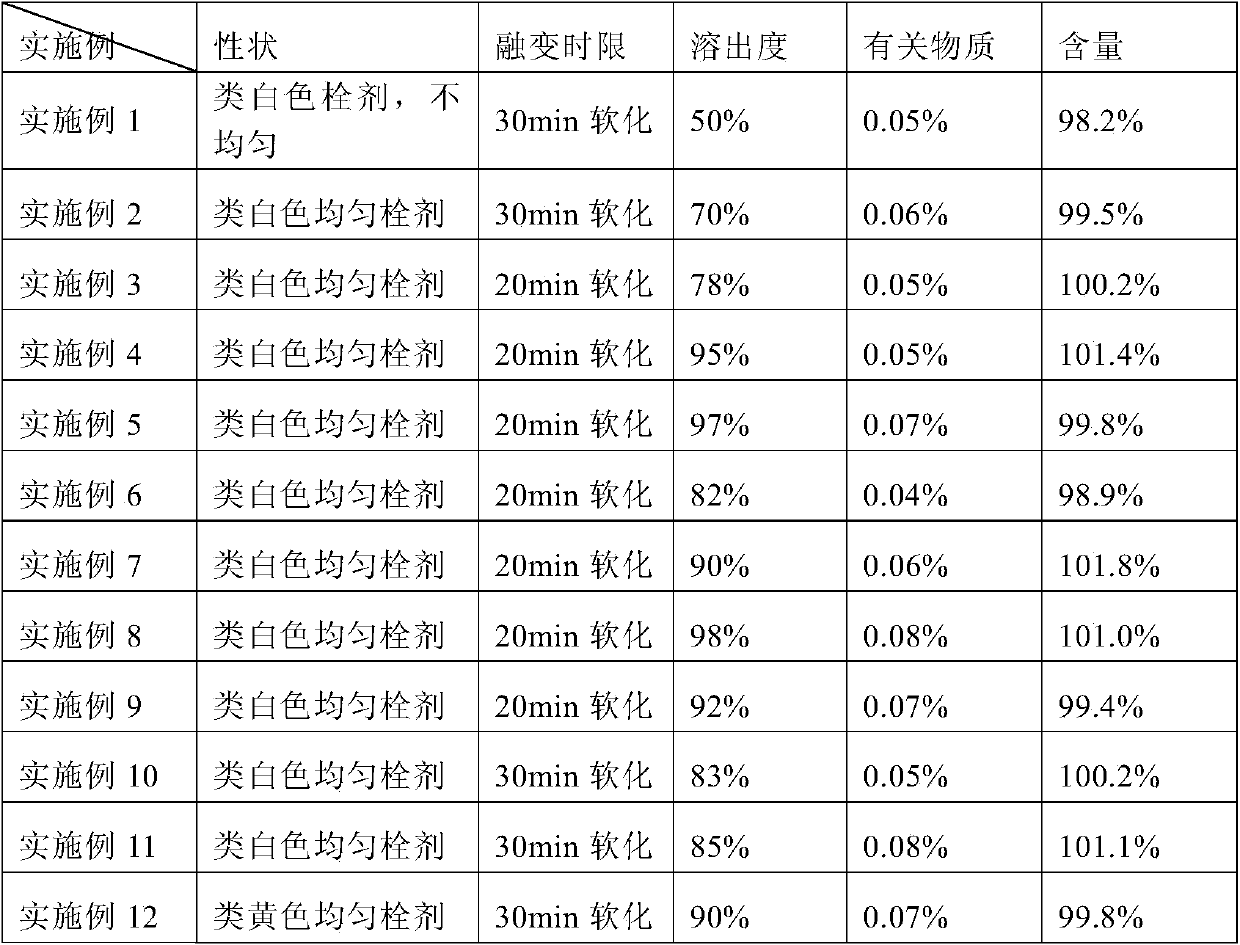
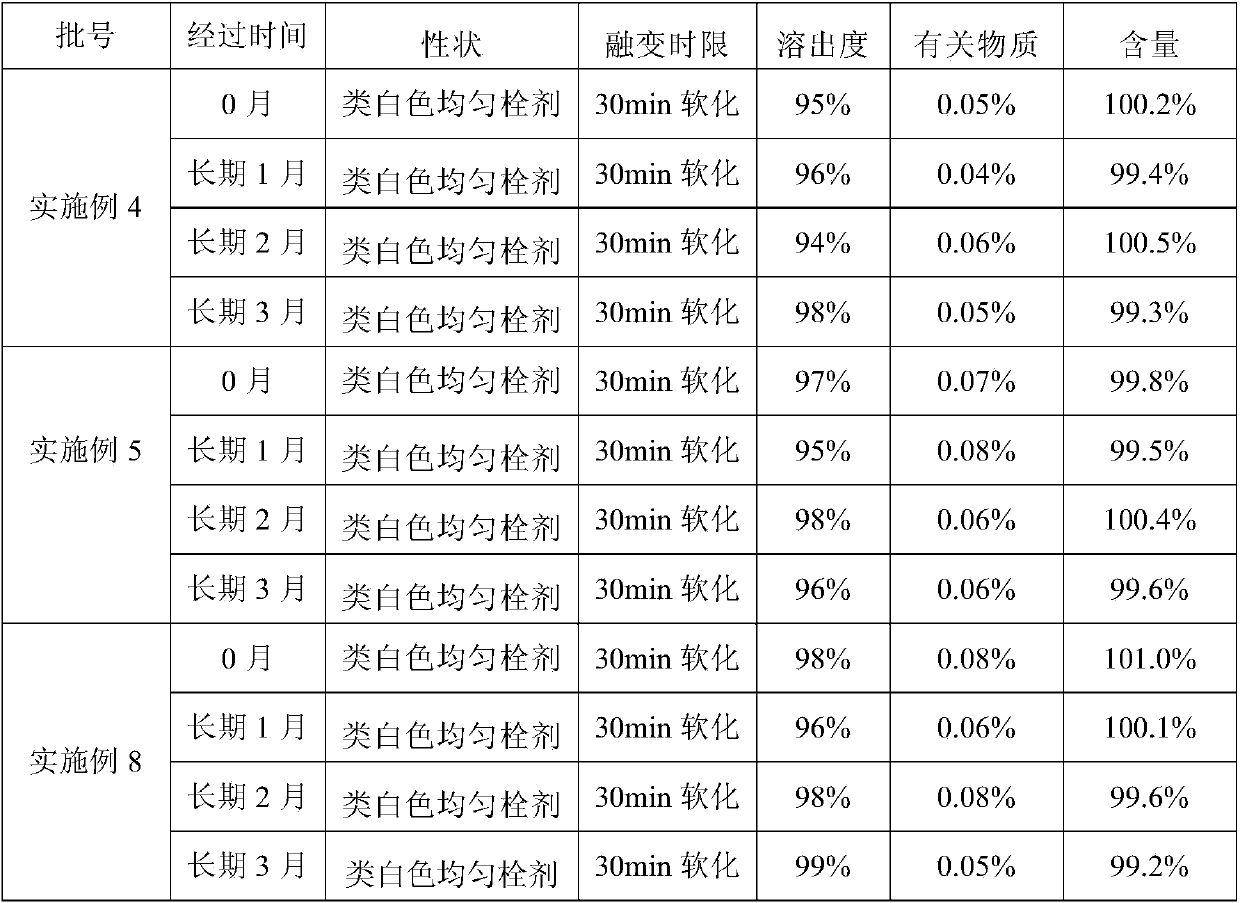
Examples
Embodiment 1
[0033] Weigh 0.35 g of Liensinine (extracted and refined in the laboratory), and heat 99.5 g of semi-synthetic mixed fatty acid ester (type 36, purchased from Lutianhua, Chengdu) and 0.1 g of Tween-80 (purchased from Sichuan Jinshan Pharmaceutical) at 50 °C After melting, add Liensinine, stir to make it evenly mixed, pour it into a preheated plug mold, cool it at 2 to 8°C for 1 to 3 hours, and then take it out. Each plug weighs 1.0 g.
Embodiment 2
[0035] Weigh 0.35 g of Liensinine (extracted and refined in the laboratory), micronized 1 to 3 times with a pulverizer, passed through a 100-mesh sieve, and mixed 99.5 g of semi-synthetic mixed fatty acid ester (model 36, purchased from Lutianhua, Chengdu) and vomited. After 0.1 g of Wen-80 (purchased from Sichuan Jinshan Pharmaceutical) was heated and melted at 50 °C, micronized Liensinine was added, stirred to make it evenly mixed, poured into a preheated plug mold, and cooled at 2 to 8 °C for 1 to 3 Take it out after an hour, and each plug weighs 1.0g.
Embodiment 3
[0037] Weigh 1.0 g of Liensinine (extracted and refined in the laboratory), micronized 1 to 3 times with a pulverizer, passed through a 100-mesh sieve, and mixed 98.5 g of semi-synthetic mixed fatty acid esters (model 34, purchased from Lutianhua, Chengdu) and spun out. After 0.5 g of Wen-80 (purchased from Sichuan Jinshan Pharmaceutical) was heated and melted at 50°C, Liensinine was added, stirred to make it evenly mixed, poured into a preheated plug mold, cooled at 2-8°C for 1-3 hours and taken out That is, each plug weighs 1.0g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com