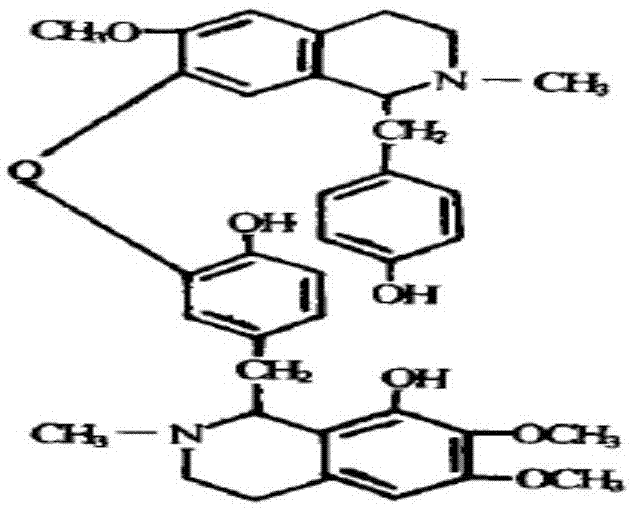
Liensinine suppository and preparation method and purpose thereof
A liensinine suppository and technology of liensinine are applied in the directions of suppository delivery, pharmaceutical formulation, cardiovascular system diseases, etc., and can solve the problems of no commercial listing of liensinine, serious liver first-pass effect, and the inability to achieve expected ideal pharmacological activity of liensinine , to avoid the effect of poor oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
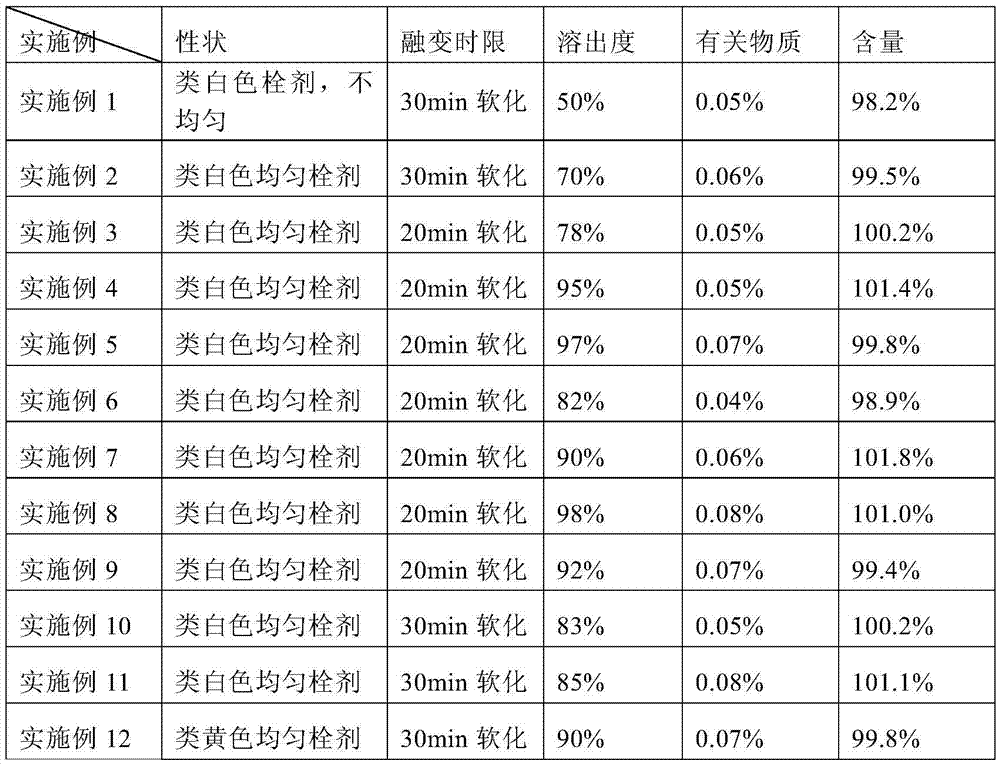
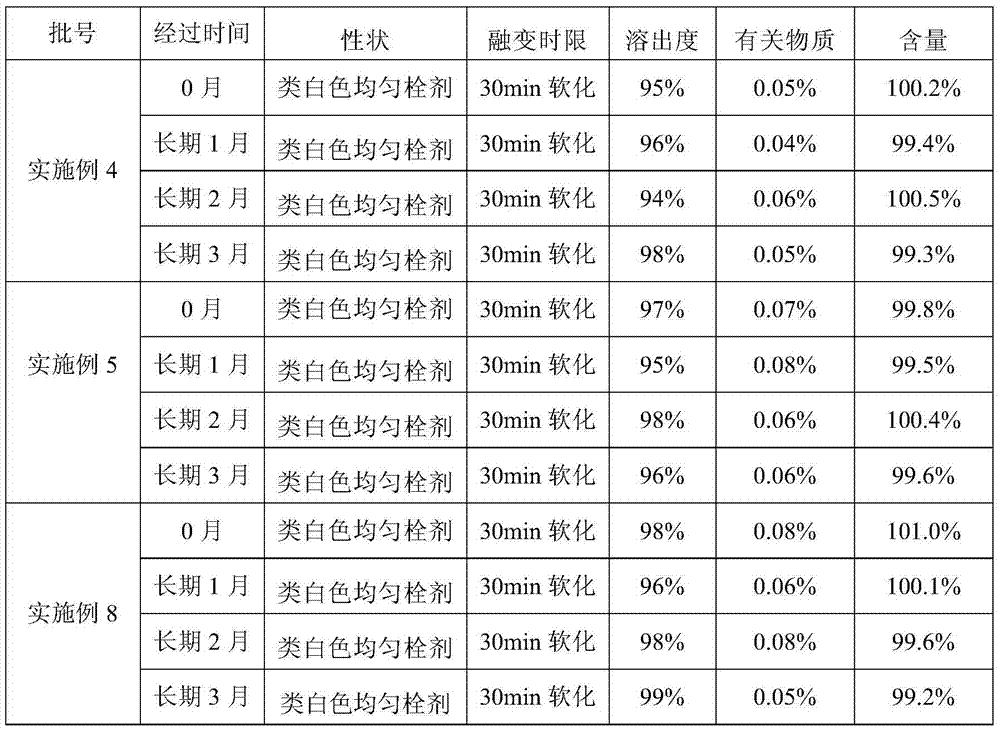
Embodiment 1
[0033] Weigh 0.35 g of liensinine (extracted and refined in the laboratory), 99.5 g of semi-synthetic mixed fatty acid lipids (model 36, purchased from Chengdu Lutianhua) and 0.1 g of Tween-80 (purchased from Sichuan Jinshan Pharmaceutical) were heated at 50 ° C After melting, add liensinline, stir to make it evenly mixed, pour into the preheated plug mold, cool at 2-8°C for 1-3 hours, and then take it out. Each plug weighs 1.0g.
Embodiment 2
[0035] Weigh 0.35 g of liensinine (extracted and refined in the laboratory), micronize 1 to 3 times with a pulverizer, pass through a 100-mesh sieve, and mix 99.5 g of semi-synthetic mixed fatty acid lipids (model 36, purchased from Chengdu Lutianhua) and spit After heating and melting 0.1g of Wen-80 (purchased from Sichuan Jinshan Pharmaceutical) at 50°C, add micronized liensinine, stir to make it evenly mixed, pour it into a preheated plug mold, and cool at 2-8°C for 1-3 Take it out after 1 hour, and each plug weighs 1.0g.
Embodiment 3
[0037] Weigh 1.0 g of liensinine (extracted and refined in the laboratory), micronize 1 to 3 times with a pulverizer, pass through a 100-mesh sieve, and mix 98.5 g of semi-synthetic mixed fatty acid lipids (model 34, purchased from Chengdu Lutianhua) into Wen-80 (purchased from Sichuan Jinshan Pharmaceutical) 0.5g was heated and melted at 50°C, added liensinline, stirred to mix evenly, poured into a preheated plug mold, cooled at 2-8°C for 1-3 hours, and then taken out That is, each bolt weighs 1.0g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com