Substituted tetraphenyl-silicon alkoxyl dibenzothiophene based derivative, and preparation method for derivative
A technology of alkoxy dibenzothiophene and tetraphenyl silicon, which is applied in the field of organic electroluminescent display materials and preparation, can solve the problems of no contribution to electroluminescence, achieve good thermal stability, improve charge transport performance, Reduces the effects of agglomeration and interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
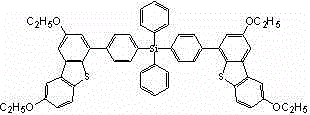
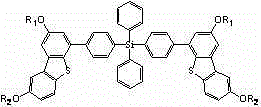
[0030] Example 1, a tetraphenyl silicon-substituted alkoxydibenzothiophene derivative, the structure of which is shown in Formula I:
[0031]
[0032] where R 1 =R 2 =CH 3 、C 2 h 5 、C 3 h 7 Any one of them, in this example R 1 =R 2 =CH 3 , with the following structure:
[0033]
[0034] The preparation route is as follows:
[0035] .
[0036] The preparation of intermediate II: under the protection of argon, add 74.2g of bis(4-bromophenyl)-phenylsilane and 50ml of tetrahydrofuran to the three-necked flask successively, cool to -78°C, add 225mL of n-butyllithium dropwise, After the dropwise addition was completed, stir at -78°C for 1 hour, then slowly add 124.28 g of tributyl borate dropwise, after the dropwise addition, keep warm for 1 hour, then automatically heat up and react overnight. Add 400ml of water, 400ml of concentrated hydrochloric acid, and 550ml of petroleum ether into the reaction flask, and stir for 2h. The organic layer was washed twice wit...
Embodiment 2
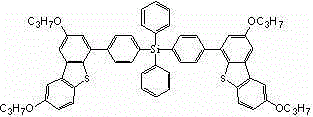
[0044] Example 2, a tetraphenyl silicon-substituted alkoxydibenzothiophene derivative, the structure of which is shown in Formula I:
[0045]
[0046] where R 1 =R 2 =CH 3 、C 2 h 5 、C 3 h 7 Any one of them, in this example R 1 =R 2 =C 2 h 5 , the structural formula is as follows:
[0047]
[0048] The preparation route is as follows:
[0049] .
[0050] The preparation of intermediate II: under the protection of argon, add 163.2g of bis(4-bromophenyl)-phenylsilane and 110ml of tetrahydrofuran to the three-necked flask successively, cool to -78°C, add 495mL of n-butyllithium dropwise, After the dropwise addition is completed, stir at -78°C for 1 hour, then slowly add 313.0 g of tributyl borate dropwise, after the dropwise addition is completed and keep warm for 1 hour, the temperature rises automatically, and the reaction lasts overnight. Add 880ml of water, 880ml of concentrated hydrochloric acid, and 1000ml of petroleum ether into the reaction flask, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com