Preparation method of cauliflower-shaped brookite type titanium dioxide
A titanium dioxide, brookite-type technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., can solve the problems of poor repeatability, short preparation period, low reaction temperature, etc., and achieve rich source of raw materials, short reaction period, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add deionized water and titanium trichloride to the polytetrafluoroethylene lining of the reaction kettle to obtain 20 ml of a 0.8 mol / L titanium trichloride solution. Then, ammonia water was added to adjust the pH to 11. Finally, 10 ml of 2.5 mol / L sodium borohydride solution was added, and after stirring evenly, the mixture was placed in a high-temperature and high-pressure reactor, and placed in an oven at 250°C for 18 hours of reaction.
[0037]After the reactor was cooled to room temperature, the supernatant was absorbed and discarded. The lower precipitate was washed 6 times with deionized water and once with absolute ethanol. After washing, the precipitate was put into an oven at 60° C. for 12 hours to be vacuum-dried to obtain a cauliflower-shaped brookite-type titanium dioxide material.
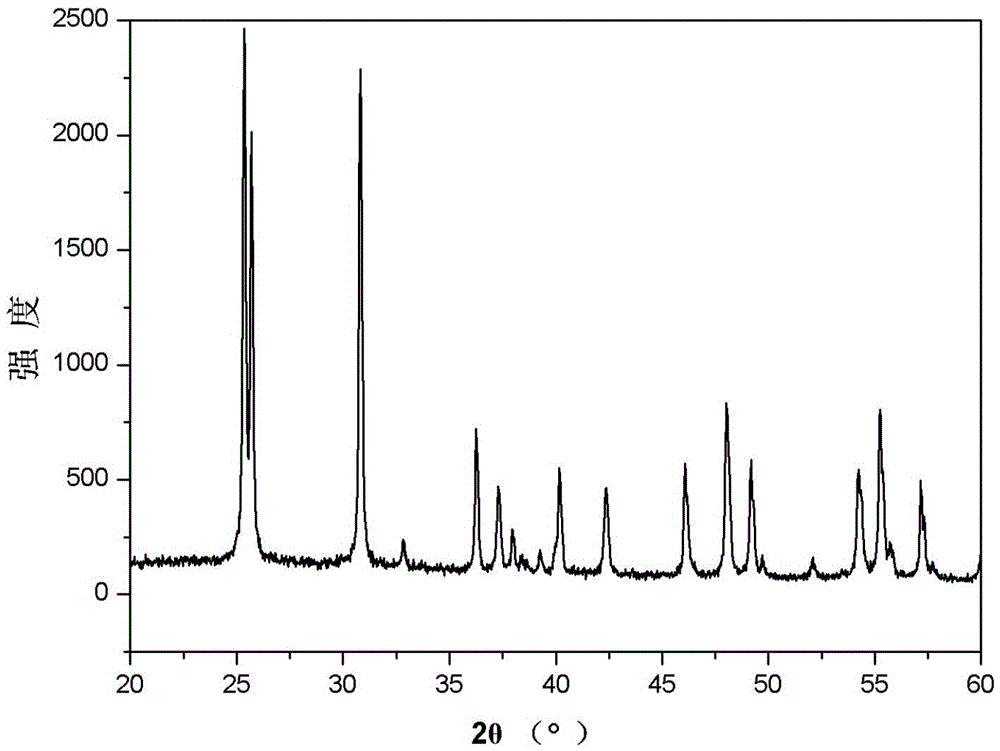
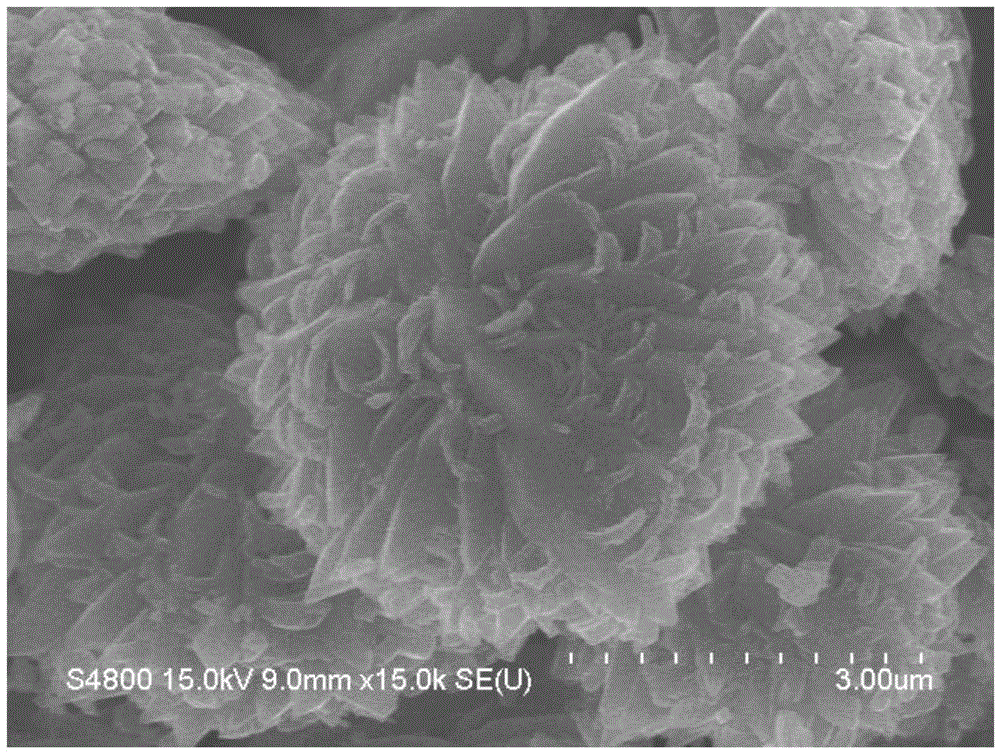
[0038] The scanning electron microscope figure of embodiment 1 gained product is as figure 2 As shown, it can be seen from the figure that the resulting product is a titan...
Embodiment 2
[0040] Add deionized water and titanium trichloride to the polytetrafluoroethylene lining of the reaction kettle to obtain 20 ml of a 0.8 mol / L titanium trichloride solution. Then, ammonia water was added to adjust the pH to 11. Finally, 10 ml of 2.5 mol / L sodium borohydride solution was added, stirred evenly, and the mixture was placed in a high-temperature and high-pressure reactor, and put into an oven at 250°C for 12 hours of reaction.
[0041] After the reactor was cooled to room temperature, the supernatant was absorbed and discarded. The lower precipitate was washed 6 times with deionized water and once with absolute ethanol. After washing, put the precipitate into a 60°C oven for vacuum drying for 12 hours to obtain a cauliflower-shaped brookite-type titanium dioxide material. image 3 is the scanning electron microscope image of the sample.
Embodiment 3
[0043] Add deionized water and titanium trichloride to the polytetrafluoroethylene lining of the reaction kettle to obtain 20 ml of a 0.8 mol / L titanium trichloride solution. Then, ammonia water was added to adjust the pH to 11. Finally, 10 ml of 2.5 mol / L sodium borohydride solution was added, stirred evenly, and the mixed solution was placed in a high-temperature and high-pressure reactor, and placed in an oven at 250°C for 24 hours of reaction.
[0044] After the reactor was cooled to room temperature, the supernatant was absorbed and discarded. The lower precipitate was washed 6 times with deionized water and once with absolute ethanol. After washing, put the precipitate into a 60°C oven for vacuum drying for 12 hours to obtain a cauliflower-shaped brookite-type titanium dioxide material. Figure 4 is the scanning electron microscope image of the sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com