Adriamycin co-drug-loading system and preparing method and application thereof
A technology of co-loading and doxorubicin, which is applied in pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., can solve the problems of easy generation of drug resistance, strong toxicity, and low selectivity of doxorubicin liposomes. Fast onset, reduced toxicity, increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the doxorubicin co-loading system provided by the invention comprises the following steps:
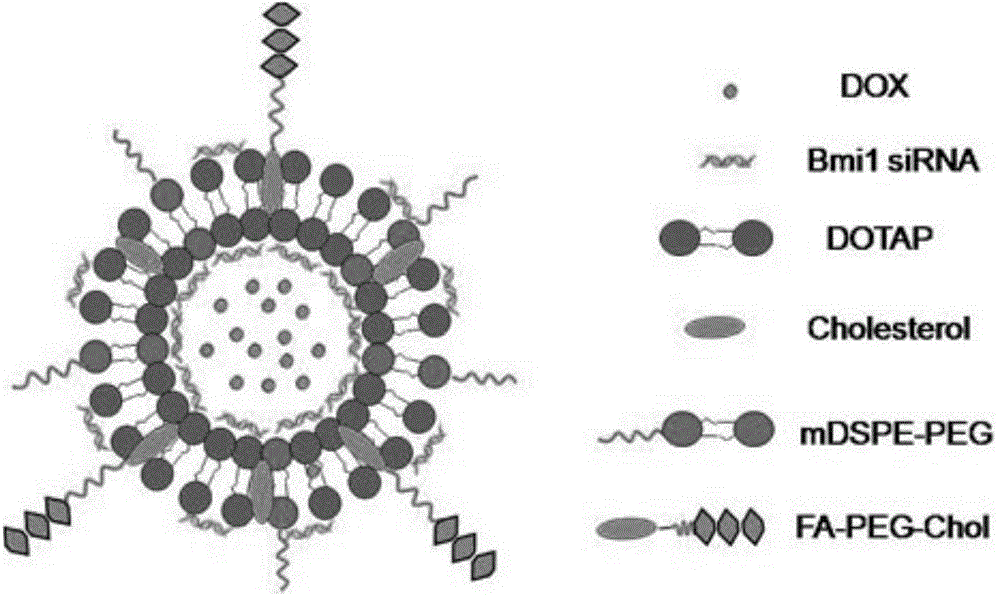
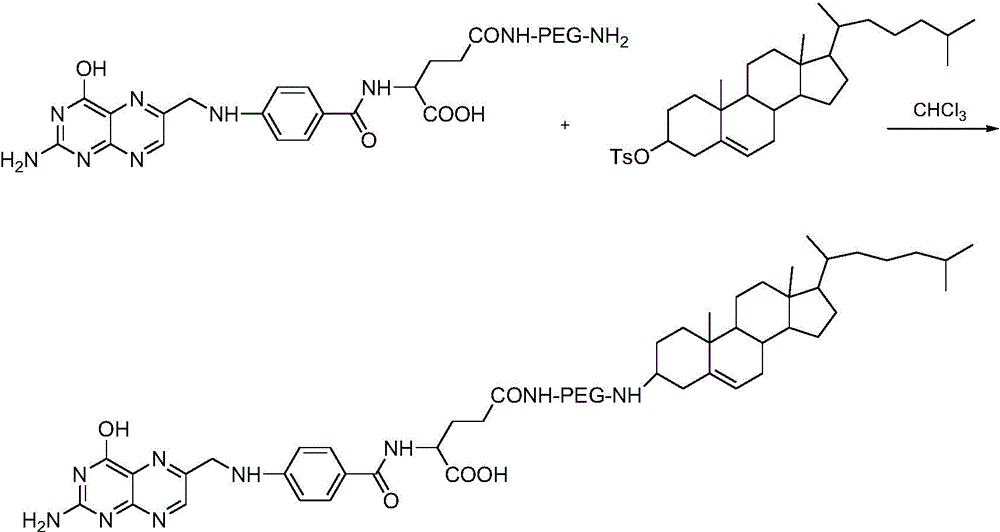
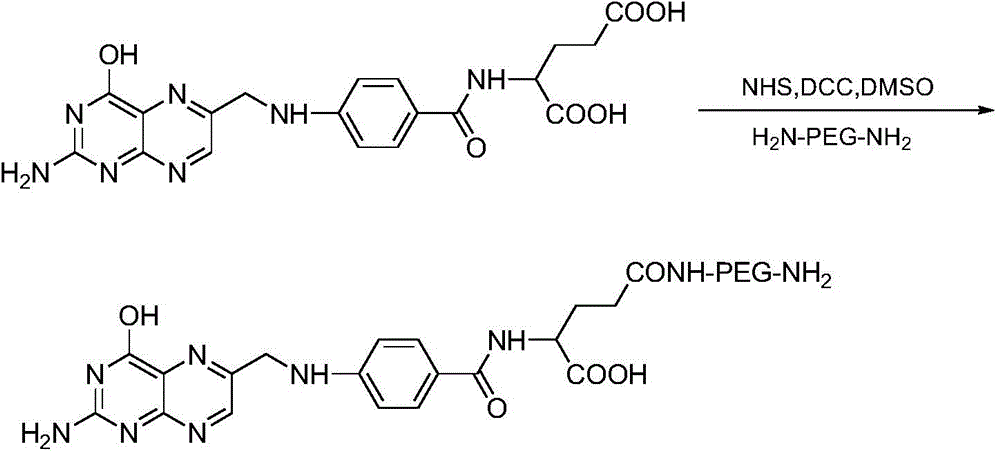
[0044] (1) Preparation of targeted conjugates of folic acid ligands: folic acid-polyethylene glycol diamine obtained by covalently linking folic acid with polyethylene glycol diamine, homogeneously mixed with cholesterol-p-toluenesulfonate and triethylamine Disperse in an organic solvent to obtain solution A, so that each milliliter of solution A contains folic acid-polyethylene glycol diamine between 15 μmol and 20 μmol, cholesterol between 20 μmol and 25 μmol, and triethylamine between 35 μmol and 45 μmol; Evaporate solution A to dryness to obtain oily substance B, add oily substance B to 40mM to 60mM sodium carbonate solution, each milliliter of sodium carbonate solution corresponds to 15mg to 60mg of oily substance B, after mixing, centrifuge to take supernatant C, supernatant C dialyzed and freeze-dried to obtain the targeting conjugate, folic acid...
Embodiment 1
[0057] A doxorubicin co-loading system, comprising the active ingredient of doxorubicin and a targeting carrier; the targeting carrier has a targeting ligand covalently bound to its surface and carries small interfering RNA for inhibiting the expression of oncogenes The doxorubicin active ingredient accounts for 4% of the mass percentage of the co-loading system; the targeting ligand accounts for 1% of the mass percentage of the co-loading system; the small interfering RNA accounts for the co-loading system The mass percentage of the medicine system is 0.5%.
[0058] The targeting ligand is preferably folic acid polyethylene glycol cholesterol ligand.
[0059] The target gene of the small interfering RNA is the driver gene of cancer, the Bmi1 gene, whose sequence is:
[0060] Sense strand: 5'-CCAGACCACUACUGAAUAUAA-3';
[0061] Antisense strand: 5'-UUAUAUUCAGUAGUGGUCUGGUU-3'.
[0062] The targeting carrier is preferably a liposome.
[0063] When the particle size of the dox...
Embodiment 2
[0084] A doxorubicin co-loading system, comprising the active ingredient of doxorubicin and a targeting carrier; the targeting carrier has a targeting ligand covalently bound to its surface and carries small interfering RNA for inhibiting the expression of oncogenes The doxorubicin active ingredient accounts for 6% of the mass percentage of the co-loading system; the targeting ligand accounts for 5% of the mass percentage of the co-loading system; the small interfering RNA accounts for the co-loading system The mass percentage of the medicine system is 1%.
[0085] The targeting ligand is preferably folic acid polyethylene glycol cholesterol ligand.
[0086] The target gene of the small interfering RNA is the driver gene of cancer, the Bmi1 gene, whose sequence is:
[0087] Sense strand: 5'-CCAGACCACUACUGAAUAUAA-3';
[0088] Antisense strand: 5'-UUAUAUUCAGUAGUGGUCUGGUU-3'.
[0089] The targeting carrier is preferably a liposome.
[0090] The particle size of the doxorubici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com