Method for preparing ether compound
A kind of ether compound, the technology of compound, is applied in the field of preparing ether compound represented by the following formula, can solve the problem such as being difficult to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
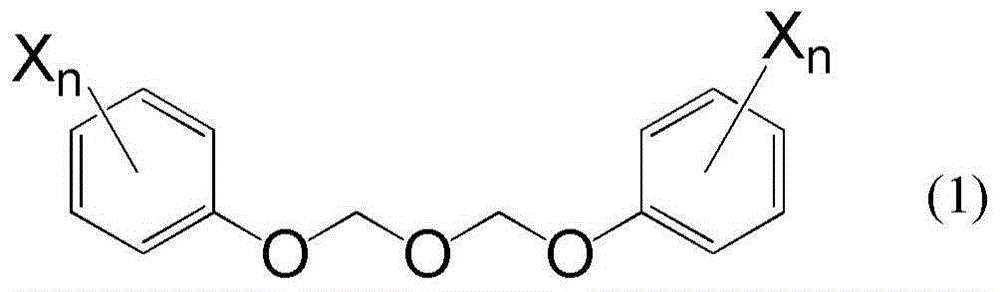
[0048] The preparation of the compound represented by the formula (1) is described below.
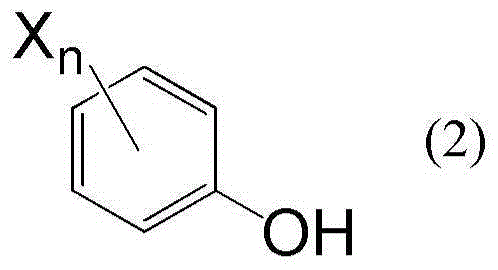
[0049] The compound represented by the formula (1) can be prepared by reacting bis(acetoxymethyl) ether with the compound represented by the formula (2):
[0050]
[0051] where n and X have the same meaning as above.
[0052] The reaction is usually carried out in an organic solvent in the presence of a metal catalyst.
[0053]Examples of the compound represented by the formula (2) include phenol, 4-chlorophenol, 4-nitrophenol, 4-cyanophenol, 2,4-dichlorophenol, 2,4,6-trichlorophenol, 2, 3,6-Trichlorophenol, 2,3,5,6-Tetrachlorophenol, 2,3,4,6-Tetrachlorophenol, 2,3,4,5,6-Pentachlorophenol, 2,6- Dichloro-4-nitrophenol, 2,6-dichloro-4-cyanophenol, 2,4,6-tribromophenol, 2,3,4,5,6-pentabromophenol, 2,6- Dibromo-4-cyanophenol and 2,3,4,5,6-pentafluorophenol.
[0054] Examples of the organic solvent include toluene, xylene, mesitylene, dichlorobenzene, and o-dichlorobenzene. Preferen...
Embodiment
[0064] Hereinafter, the present invention will be further specifically described with reference to Examples and the like.
[0065] First, the preparation example of the ether compound is described.
preparation example 1-1
[0066] Preparation Example 1-1 (Steps A to C)
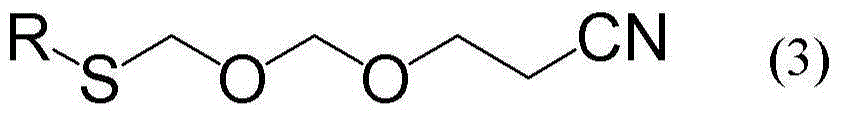
[0067] To 800 ml of 2-methoxyethanol, 100 g (purity 95.0 g) of bis(2,4,6-trichlorophenoxymethyl) ether containing 5 wt% alumina and 50% water were added of 91.4 g (pure content 45.7 g, 3.0 equiv.) of sodium methanethiolate, followed by stirring at 100 to 105° C. for 4 hours under nitrogen atmosphere. The reaction mixture was cooled to room temperature and filtered. Thereafter, hexane and 10% aqueous NaOH solution were added, followed by stirring. The mixture was left to stand and then separated. The obtained organic solvent layer was dried over anhydrous sodium sulfate, and then the solvent was distilled off under normal pressure, whereby 32.2 g of bis(methylthiomethyl)ether (referred to as compound Y) was obtained as a residue. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com