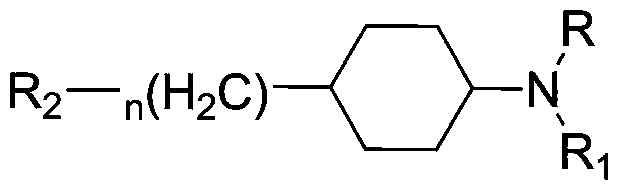Piperazine (pyridine) cyclohexyl derivatives and their application in the treatment of mental and nervous diseases
A technology of pyridine cyclohexyl derivatives, which is applied in the field of piperazine cyclohexyl derivatives, can solve the problems of cognitive impairment and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
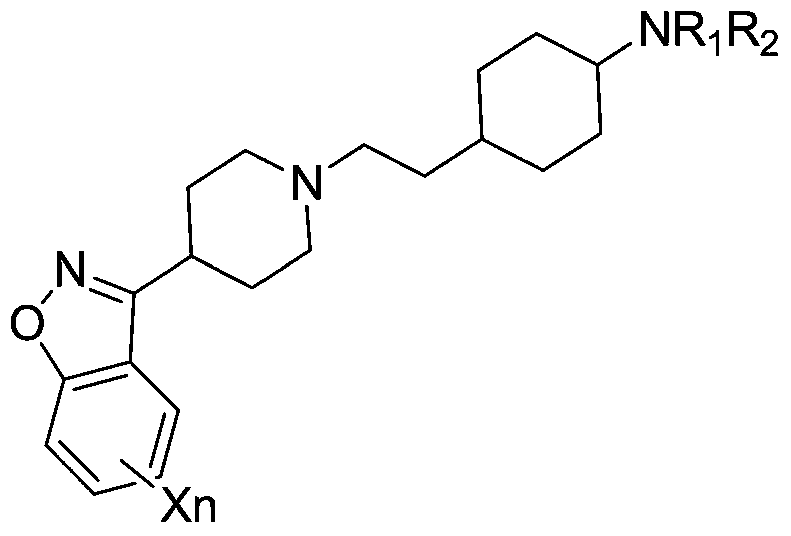
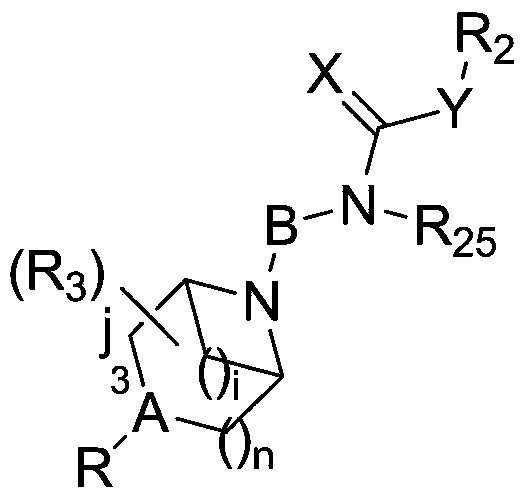
[0178] Trans-N-(4-(2-(4-(Benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)oxazol-2-amine (I-1 ) and its salt preparation
[0179]Trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amine (prepared according to General Method 7) ( 3.45g, 10mmol), 2-chlorooxazole (1.04g, 10mmol) were added to acetonitrile (50mL), (DIPEA, 50mmol) was added dropwise, and the reaction was refluxed for 12h. The reaction solution was cooled to room temperature, and a white solid was precipitated, which was filtered to obtain The crude product was recrystallized from 95% ethanol to obtain 2.42 g of white solid with a yield of 58.7%.
[0180] 1 HNMR (DMSO-d 6 ,δ:ppm):0.95-1.04(m,2H,A-H),1.18-1.27(m,3H,A-H),1.38-1.40(m,2H,A-H),1.75-1.77(m,2H,A-H), 1.87-1.91(m,2H,A-H),2.40(t,2H,J=7.8Hz,N-CH 2 ),2.61-2.66(m,4H,piperazine-CH 2 ),3.43-3.48(m,4H,piperazine-CH 2 ),3.60-3.67(m,1H,A-H),6.82(d,1H,J=2.4Hz,Ar-H),7.02(d,1H,J=8.0Hz,NH-H)7.43(t,1H, J=7.6Hz, Ar-H), 7.55(t, 1H, J=7....
Embodiment 2
[0189] 3-(trans-4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)oxazolidin-2-one (I- 14) Preparation of its salt
[0190] Trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amine (prepared according to General Method 7) ( 3.45g, 10mmol) and bromoethanol (1.25g, 10mmol) were added to dichloromethane (50mL), refluxed for 48h, the reaction solution was cooled to room temperature, and washed once with saturated sodium carbonate solution, water, and saturated brine successively. The organic phase was concentrated to dryness under reduced pressure to obtain 2-(trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclo Hexyl)amino)ethanol crude product. Dissolve triphosgene (0.74g, 2.5mmol) in dichloromethane (20mL), and add 2-(trans-N-(4-(2-(4-(benzo[d] Isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amino)ethanol (1.94g, 5mmol), triethylamine (12.5mmol) in dichloromethane (10mL) solution, stirring reaction at room temperature Af...
Embodiment 3
[0200] 3-(trans-4-(2-(4-(benzo[d]isoxazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)quinazoline-2,4(1H ,3H)-diketone (I-15) and its salt preparation
[0201] Trans-N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amine (prepared according to General Method 7) ( 3.45g, 10mmol) and isatoic anhydride (1.48g, 9.1mmol) were added to dichloromethane (50mL), reacted at an external temperature of 50°C for 12h, added triphosgene (9.1mmol), potassium carbonate (45.5mmol), and reacted for 12h , to stop the reaction, the reaction solution was cooled to room temperature, and washed once with saturated ammonium chloride solution, water, and saturated brine successively, and the organic phase was concentrated to dryness under reduced pressure to obtain a white solid, which was separated by a Flash column to obtain 3.1 g of a white solid, and was collected. The rate is 69.4%.
[0202] 1 HNMR (DMSO-d 6 ,δ:ppm):1.03-1.09(m,2H,A-H),1.23-1.44(m,4H,A-H),1.58-1.61(m,2H,A-H),1.84-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com