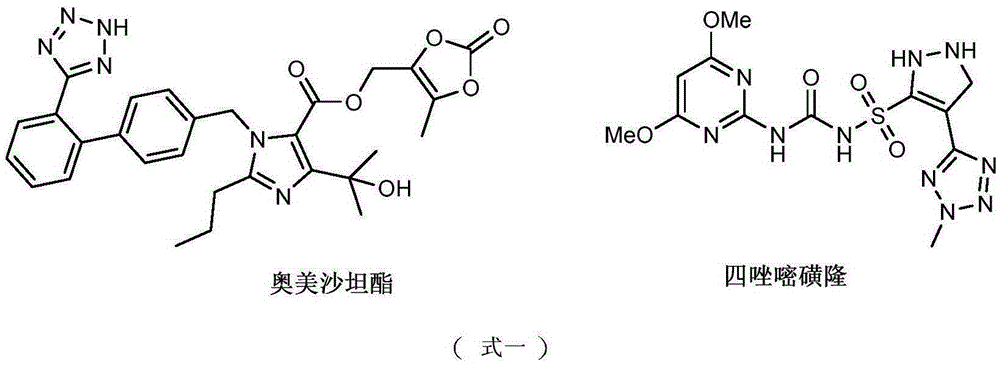
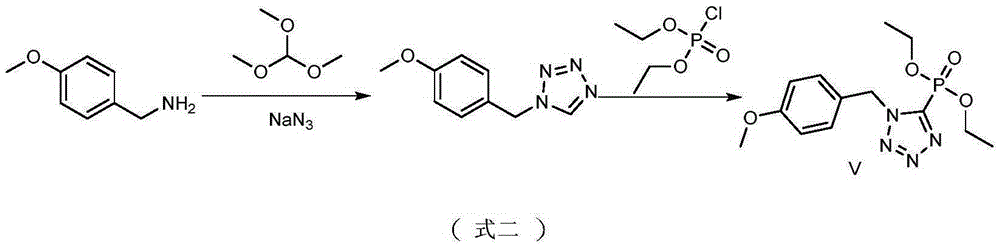
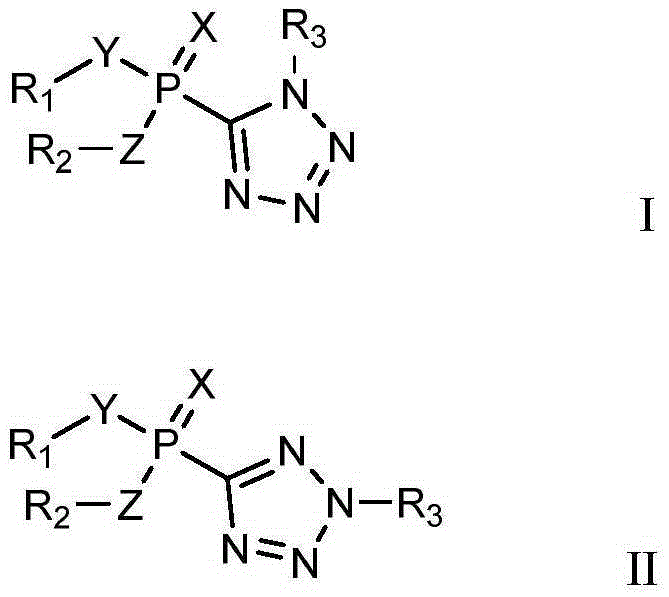
Organophosphorus compounds containing tetrazole heterocycle and synthetic method and application of organophosphorus compound
A synthetic method and technology of azole compounds, which are applied in the field of organic compound synthesis, can solve problems such as easy degradation, high toxicity, and short residual effect period, and achieve the effect of improving the efficiency of activity screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Intermediate 1-Benzyl-1H-tetrazole III-1
[0030]
[0031] Add 1mL of N,N-dimethylformamide, tetrazole (2mmol), benzyl chloride (2.1mmol) and anhydrous potassium carbonate (3mmol) to a dry reaction flask with a thermometer, and slowly heat to 100°C under stirring React overnight. The reaction was cooled to room temperature and poured into 4 ml of water. Transfer to a separatory funnel, extract with ethyl acetate, collect the organic phase, wash with water, wash with brine, dry, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain the crude product of the mixture of III-1 and IV-1, which was precipitated by recrystallization or purified by silica gel column with ethyl acetate:petroleum ether=1:5 (V / V) to obtain white solid III-1, which was recovered rate 43%. 1 HNMR (400 MHz, Chloroform-d) δ 8.56 (d, J = 5.5 Hz, 1H), 7.51 - 7.25 (m, 6H), 5.61 (s, 2H).
Embodiment 2
[0032] Example 2: 1-(2-chlorobenzyl)-1H-tetrazolyl-diethyl phosphate
[0033]
[0034] Under nitrogen protection, 1-benzyltetrazole (1 mmol) was added to the dry reactor, and 10 mL of dry THF was added. Cool down to below -10°C, and slowly add butyllithium (2.2mmol, 1M, 1.1eq) dropwise. After the dropwise addition, react at this temperature for 1 hour, and then add diethyl chlorophosphate (1mmol, 1eq) dropwise. After the dropwise addition, this temperature was maintained for 1 hour. Rising to room temperature, stirring for 1 hour, the raw materials were completely reacted. Washed twice with water and dried over anhydrous magnesium sulfate. Filtrate, spin and concentrate the solvent to obtain a crude product, which is purified by a silica gel column with ethyl acetate:petroleum ether=1:8 (V / V), and the yield is 91%. 1HNMR (400MHz, Chloroform-d) δ7.50–7.33(m, 5H).4.49(d, J=8.7Hz, 2H), 4.28–4.03(m, 4H), 1.34(td, J=7.1, 1.2Hz , 6H). 13 CNMR (101MHz, CDCl 3 )δ134.48, 134....
Embodiment 3
[0035] Example 3: 1-(2-chlorobenzyl)-1H-tetrazolyl-diethyl phosphate
[0036]
[0037] Add 1mL of N,N-dimethylformamide, tetrazole (2mmol), 2-chlorobenzyl (2.1mmol) and anhydrous potassium carbonate (3mmol) into a dry reaction flask with a thermometer, and heat slowly under stirring React overnight at 100°C. The reaction was cooled to room temperature and poured into 4 ml of water. Transfer to a separatory funnel, extract with ethyl acetate, collect the organic phase, wash with water, wash with brine, dry, and filter. The filtrate was concentrated to dryness under reduced pressure to obtain the crude product of the mixture of III-2 and IV-2 with a yield of 95%.
[0038] Under nitrogen protection, the crude product of the mixture of III-2 and IV-2 was added to a dry reactor, and 10 ml of dry tetrahydrofuran was added. Cool down to below -10°C, and slowly add butyllithium (2.2mmol, 1M, 1.1eq) dropwise. After the dropwise addition was completed, the reaction was carried ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com