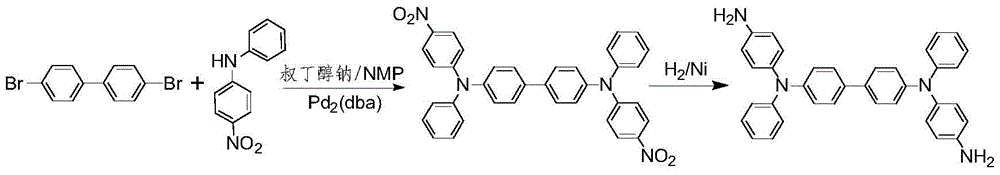
A kind of preparation method of oled intermediate material triphenylamine derivatives
A technology of bitriphenylamine and intermediates, which is applied in the field of chemical synthesis, can solve the problems of large amount of catalyst, high reaction temperature and high cost, and achieves the effects of reducing the cost of raw materials, reducing the reaction temperature and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
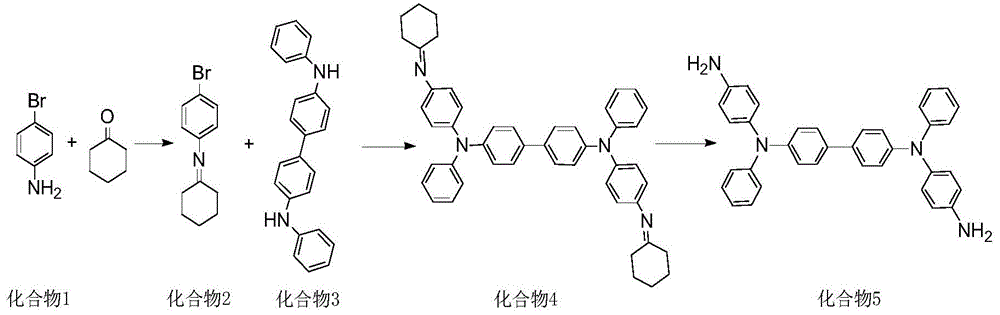
[0026] Example 1: Preparation of N4, N4'-bis(4-aminophenyl)-N4, N4'-diphenyl-[1,1'-biphenyl]-4,4'-diamine (compound 5)
[0027]
[0028] Synthesis of compound 2:
[0029] In a 250ml round bottom flask, add p-bromoaniline (13.8g, 80mmol), cyclohexanone (7.8g, 80mmol), p-toluenesulfonic acid (5.5g, 32mmol), toluene 180ml, under nitrogen protection, heat up to 100°C , stirred for 15h (TLC tracking detection, until the raw material point disappeared). After the reaction was cooled, it was washed with water until pH = 7, concentrated by rotary evaporation to obtain a deep red solid, purified by crystallization with toluene, and dried in vacuo to obtain 18.8 g of a deep red solid with a yield of 93.5%.
[0030] Synthesis of compound 4:
[0031] In a 250ml round bottom flask, add compound 2 (17.6g, 70mmol), compound 3 (10.8g, 32mmol), potassium tert-butoxide (11.2g, 100mmol), tetrakistriphenylphosphine palladium (0.185g, 0.16mmol ), DMF180ml, under the protection of nitrogen, s...
Embodiment 2
[0034] Example 2: Preparation of N4, N4'-bis(4-aminophenyl)-N4,N4'-bis(3-methylphenyl)-[1,1'-biphenyl]-4,4'- Diamine (Compound 10)
[0035]
[0036] Synthesis of compound 7:
[0037] In a 250ml round bottom flask, add p-bromoaniline (13.8g, 80mmol), 9-fluorenone (14.4g, 80mmol), p-toluenesulfonic acid (5.5g, 32mmol), toluene 180ml, under nitrogen protection, heat up to 100 °C, stirred for 20 h (TLC tracking detection until the raw material point disappeared). After the reaction was cooled, it was washed with water until pH = 7, concentrated by rotary evaporation to obtain a deep red solid, purified by crystallization of toluene, and dried in vacuo to obtain 23.8 g of a deep red solid with a yield of 90%.
[0038] Synthesis of Compound 9:
[0039] In a 250ml round bottom flask, add compound 7 (23.3g, 70mmol), compound 8 (11.6g, 32mmol), potassium tert-butoxide (11.2g, 100mmol), tetrakistriphenylphosphine palladium (0.37g, 0.32mmol ), DMF180ml, under the protection of nit...
Embodiment 3
[0042] Example 3: Preparation of N4, N4'-bis(3-aminophenyl)-N4,N4'-diphenyl-[1,1'-biphenyl]-4,4'-diamine (compound 15)
[0043]
[0044] Synthesis of Compound 12:
[0045] In a 250ml round bottom flask, add m-bromoaniline (13.8g, 80mmol), cyclohexanone (7.8g, 80mmol), p-toluenesulfonic acid (5.5g, 32mmol), toluene 180ml, under nitrogen protection, heat up to 100°C , stirred for 20h (TLC tracking detection, until the raw material point disappeared). After the reaction was cooled, it was washed with water until pH = 7, concentrated by rotary evaporation to obtain a deep red solid, which was purified by toluene crystallization, and dried in vacuo to obtain 18.5 g of a deep red solid with a yield of 92%.
[0046] Synthesis of Compound 14:
[0047] In a 250ml round bottom flask, add compound 12 (17.6g, 70mmol), compound 13 (10.8g, 32mmol), potassium tert-butoxide (11.2g, 100mmol), tetrakistriphenylphosphine palladium (0.37g, 0.32mmol ), DMF180ml, under the protection of nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com