Application of Cephaloziellin F in preparation of drugs for treating chronic granulocytic leukemia
A chronic myeloid leukemia technology, applied in drug combination, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problem that there is no report of chronic myeloid leukemia activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
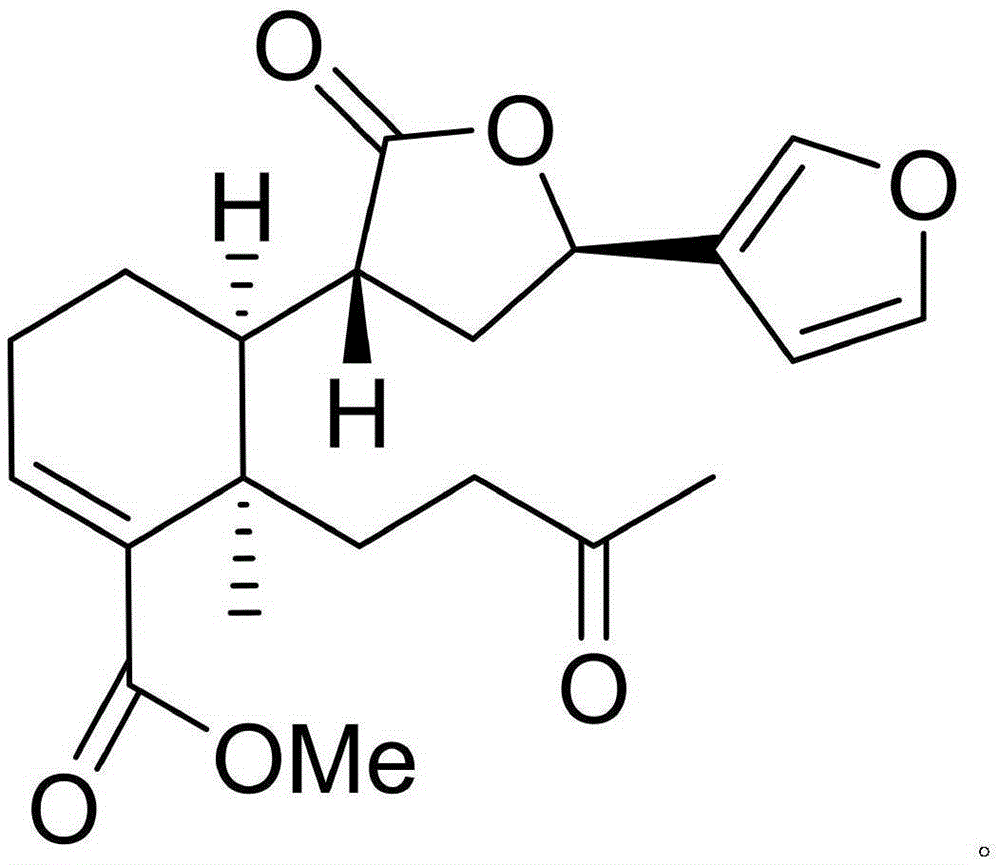
[0010] Example 1: Isolation, Preparation and Structure Confirmation of Cephaloziellin F
[0011] The preparation method of Cephaloziellin F is the same as that reported in the literature (Secondary Metabolites from the Chinese Liverwort Cephaloziellakiaeri, J. Nat. Prod., 2013, 76, 1700-1708).
[0012] Confirmation of structure: colorless oil (methanol). According to HR-ESI-MS, the molecular formula is C 21 h 26 o 6 , with an unsaturation of 9. H NMR spectrum data δ H (ppm, DMSO-d 6 , 600MHz): H-1(1.49, m), H-1(1.91, m), H-2(2.32, m), H-2(2.11, m), H-3(6.83, br, s) , H-6 (1.79, m), H-6 (2.13, m), H-7 (2.83, m), H-7 (2.22, m), H-9 (2.95, t, J=9.6), H-10 (1.53, m), H-11 (2.35, m, 2H), H-12 (5.40, br, d, J=6.6), H-14 (6.29, br, s), H-15 ( 7.35, br, s), H-16 (7.37, br, s), H-17 (2.04, s), H-19 (1.11, s), 18-OMe (3.70, s); carbon NMR data δ C (ppm, DMSO-d 6 , 150Hz): 20.8 (CH 2 , 1-C), 25.7 (CH 2 , 2-C), 139.1 (CH, 3-C), 136.2 (C, 4-C), 39.0 (C, 5-C), 29.4 (CH 2 , 6-C...
Embodiment 2
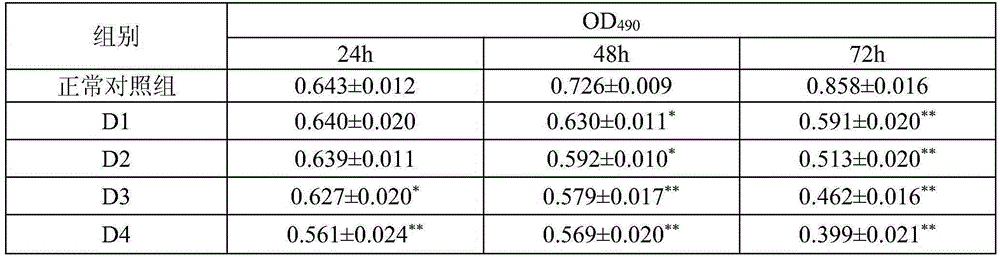
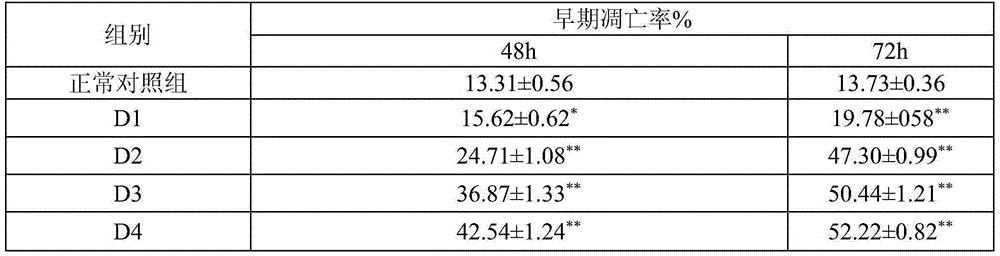
[0013] Embodiment 2: Pharmacological action test of Cephaloziellin F
[0014] 1. Materials and Instruments
[0015] K562 cells were provided by Shanghai Institute of Biological Cells, Chinese Academy of Sciences. Cephaloziellin F is self-made, and the HPLC normalized purity is greater than 98%. RPMI-1640 dry powder medium was purchased from Gibco. Calf serum was purchased from Hangzhou Sijiqing Institute of Bioengineering Materials. Penicillin and streptomycin were purchased from Huabei Pharmaceutical Factory. Glutamine, MTT, DMSO were purchased from AMRESCO. Annexinv-FITC cell apoptosis detection kit was purchased from Nanjing KGI Biotechnology Development Co., Ltd. MOPS, Rnasin, agarose, olfactory ethidium, bromophenol blue Huamei Bioengineering Company Huamei Bioengineering Company.
[0016] CO 2 Incubator (Thermo Company, USA), ultra-clean bench (Suzhou Purification Equipment Factory), magnetic stirrer (Shanghai Nanhui Telecommunication Equipment Factory), inverted ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com