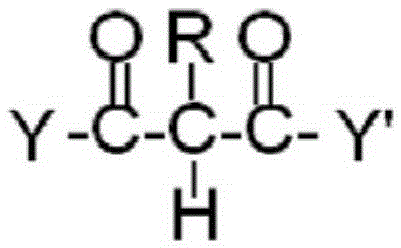
Composition crosslinkable by real michael addition (RMA) reaction
A cross-linking composition and composition technology, applied in the direction of coatings, polyester coatings, etc., can solve the problems of inapplicability of highly colored systems, inapplicability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] Preparation of malonate polyester A
[0094] Into a reactor equipped with a distillation column packed with Raschig rings, 17.31 mol of neopentyl glycol, 8.03 mol of hexahydrophthalic anhydride and 0.0047 mol of butylstannoic acid were introduced. The mixture was polymerized at 240°C in nitrogen to an acid value of 0.2 mgKOH / g. The mixture was cooled to 130° C. and 10.44 mol of diethyl malonate were added. The reaction mixture was heated to 170 °C and ethanol was removed under reduced pressure. The almost colorless material was cooled and diluted to 90% solids with 420 g butyl acetate. The final resin had an acid value of 0.3 mgKOH / g solid, an OH value of 20 mgKOH / g solid, and a weight average molecular weight of 3400 Da.
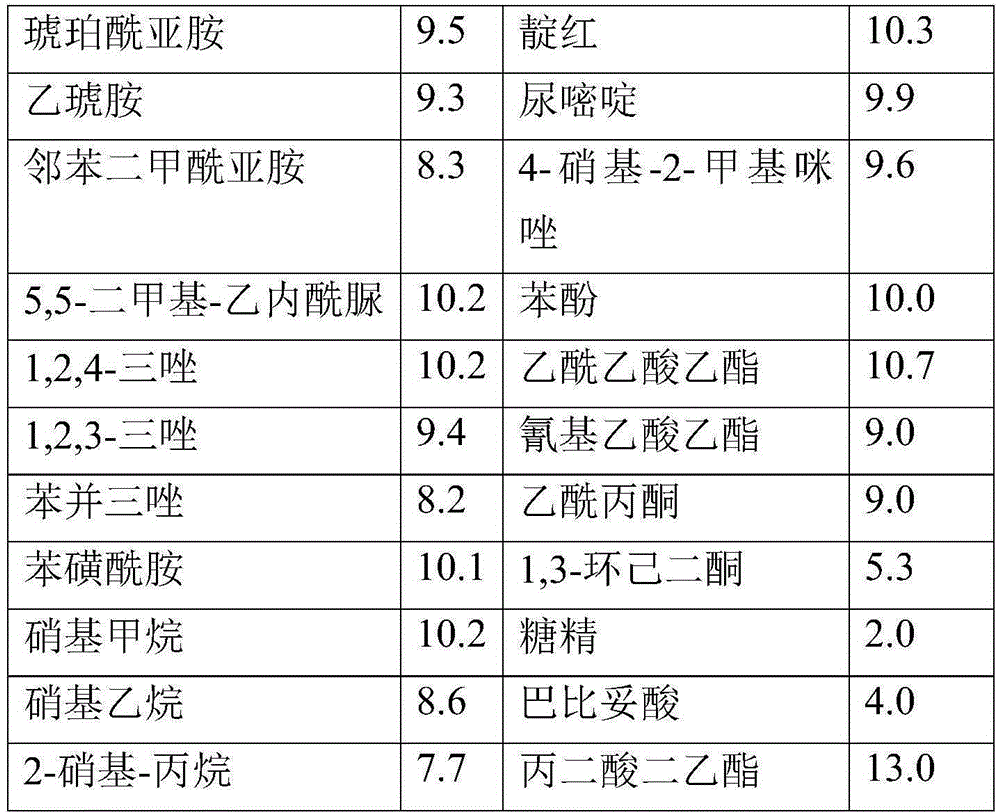
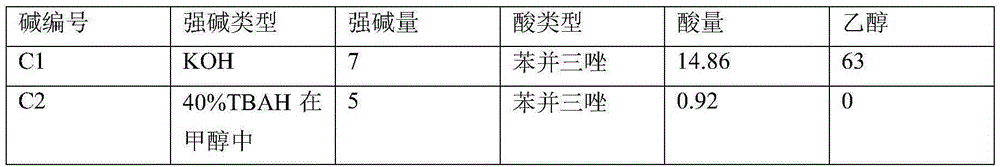
[0095] Preparation of alkaline solution C
[0096] A certain amount of acid (X-H) according to Table A was dissolved in a solution of a strong base in an alcoholic solvent (amounts in grams; all strong base / acid molar ratios equal 1). The sol...
Embodiment 18
[0125] Example 18. Determination of Michael Addition Reactivity of Succinimide
[0126] Dissolve 5 g (50.5 mmol) of succinimide in a mixture of 42 g of butyl acrylate and 42 g of methanol and either as such, or after adding a strong base (9.82 g of 1.12 meq / g tetrabutylammonium hydroxide in methanol solution, 11meq) and kept at room temperature. Subsequently, the concentration of succinimide was determined as a function of time by taking a sample, neutralizing it with a known excess of HCl in water, and back titrating it with KOH solution. Without base initiation, no appreciable loss of succinimide N-H was observed in solution over two weeks. With the addition of base, it can be seen that the concentration of succinimide decreases over time, as shown in Table F below. The succinimide concentration is expressed as % relative to the theoretical level based on the amount used.
[0127] Form F
[0128] time (minutes)
Succinimide residue (%)
3
99
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com